Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma
- PMID: 21909131
- PMCID: PMC3728834
- DOI: 10.1038/onc.2011.390
Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma
Abstract
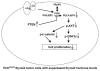
Thyroid cancers are the most common malignancy of the endocrine system in humans. To understand the molecular genetic events underlying thyroid carcinogenesis, we have generated a mouse model that spontaneously develops follicular thyroid carcinoma similar to human thyroid cancer (Thrb(PV/PV) mouse). This mutant mouse harbors a dominant-negative mutated thyroid hormone receptor β (denoted PV). The PV mutation was identified in a patient with resistance to thyroid hormone (TH). Thrb(PV/PV) mice exhibit highly elevated serum thyroid-stimulating hormone levels and increased TH. We have previously shown that thyroid-stimulating hormone is required, but not sufficient to induce metastatic follicular thyroid cancer in Thrb(PV/PV) mice. However, whether the elevated TH also contributes to the thyroid carcinogenesis of Thrb(PV/PV) mice was not elucidated. To understand the role of TH in thyroid carcinogenesis, we blocked the production of TH by treating Thrb(PV/PV) mice with propylthiouracil (Thrb(PV/PV)-PTU mice) and compared the development of thyroid cancer in Thrb(PV/PV)-PTU and untreated Thrb(PV/PV) mice. We found that thyroid tumor growth was reduced by ∼42% in Thrb(PV/PV)-PTU mice as compared with Thrb(PV/PV) mice. Analysis by bromodeoxyuridine-nuclear labeling showed decreased incorporation of bromodeoxyuridine in thyroid tumor cells of Thrb(PV/PV)-PTU mice, indicative of decreased tumor cell proliferation. However, cleaved-caspase 3 staining showed no apparent changes in apoptosis of tumor cells in Thrb(PV/PV)-PTU mice. Molecular studies identified a marked attenuation of the PI3K-AKT-β-catenin signaling pathway that led to decreased protein levels of cyclin D2, thereby decreasing tumor cell proliferation in Thrb(PV/PV)-PTU mice. Furthermore, matrix metalloproteinase-2, a downstream target of β-catenin and a key regulator during tumor invasion and metastasis, was also decreased. Thus, the present study uncovers a critical role of TH in promoting the thyroid carcinogenesis of Thrb(PV/PV) mice via membrane signaling events. Importantly, these findings suggest that anti-thyroid drugs could be considered as possible therapeutic agents of thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
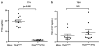
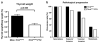


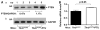

Similar articles
-
Role of TSH in the spontaneous development of asymmetrical thyroid carcinoma in mice with a targeted mutation in a single allele of the thyroid hormone-β receptor.Endocrinology. 2012 Oct;153(10):5090-100. doi: 10.1210/en.2012-1600. Epub 2012 Aug 23. Endocrinology. 2012. PMID: 22919057 Free PMC article.
-
Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma.Endocrinology. 2010 Apr;151(4):1929-39. doi: 10.1210/en.2009-1017. Epub 2010 Feb 4. Endocrinology. 2010. PMID: 20133453 Free PMC article.
-
Synergistic signaling of KRAS and thyroid hormone receptor β mutants promotes undifferentiated thyroid cancer through MYC up-regulation.Neoplasia. 2014 Sep;16(9):757-69. doi: 10.1016/j.neo.2014.08.003. Neoplasia. 2014. PMID: 25246276 Free PMC article.
-
Extranuclear signaling of mutated thyroid hormone receptors in promoting metastatic spread in thyroid carcinogenesis.Steroids. 2011 Aug;76(9):885-91. doi: 10.1016/j.steroids.2011.03.016. Epub 2011 Apr 5. Steroids. 2011. PMID: 21473875 Free PMC article. Review.
-
Nongenomic activation of phosphatidylinositol 3-kinase signaling by thyroid hormone receptors.Steroids. 2009 Jul;74(7):628-34. doi: 10.1016/j.steroids.2008.10.009. Epub 2008 Oct 30. Steroids. 2009. PMID: 19014961 Free PMC article. Review.
Cited by
-
Molecular pathogenesis and mechanisms of thyroid cancer.Nat Rev Cancer. 2013 Mar;13(3):184-99. doi: 10.1038/nrc3431. Nat Rev Cancer. 2013. PMID: 23429735 Free PMC article. Review.
-
Thyroid-specific ablation of the Carney complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer.Endocr Relat Cancer. 2012 May 24;19(3):435-46. doi: 10.1530/ERC-11-0306. Print 2012 Jun. Endocr Relat Cancer. 2012. PMID: 22514108 Free PMC article.
-
CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model.Oncogene. 2013 May 30;32(22):2726-38. doi: 10.1038/onc.2012.301. Epub 2012 Jul 16. Oncogene. 2013. PMID: 22797060 Free PMC article.
-
Thyroid Hormone Receptor β Suppression of RUNX2 Is Mediated by Brahma-Related Gene 1-Dependent Chromatin Remodeling.Endocrinology. 2018 Jun 1;159(6):2484-2494. doi: 10.1210/en.2018-00128. Endocrinology. 2018. PMID: 29750276 Free PMC article.
-
Three-dimensional telomere dynamics in follicular thyroid cancer.Thyroid. 2014 Feb;24(2):296-304. doi: 10.1089/thy.2013.0118. Epub 2013 Sep 4. Thyroid. 2014. PMID: 23819464 Free PMC article.
References
-
- Abbosh PH, Nephew KP. Multiple signaling pathways converge on beta-catenin in thyroid cancer. Thyroid. 2005;15:551–561. - PubMed
-
- Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, et al. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–2871. - PubMed
-
- Boelaert K, McCabe CJ, Tannahill LA, Gittoes NJ, Holder RL, Watkinson JC, et al. Pituitary tumor transforming gene and fibroblast growth factor-2 expression: potential prognostic indicators in differentiated thyroid cancer. J Clin Endocrinol Metab. 2003;88:2341–2347. - PubMed
-
- Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase Bmammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19:102–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

