Manganese superoxide dismutase is a mitochondrial fidelity protein that protects Polγ against UV-induced inactivation
- PMID: 21909133
- PMCID: PMC3237716
- DOI: 10.1038/onc.2011.407
Manganese superoxide dismutase is a mitochondrial fidelity protein that protects Polγ against UV-induced inactivation
Abstract
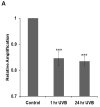
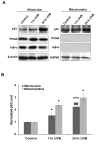
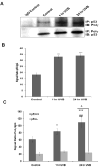
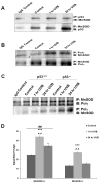
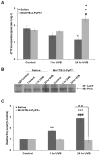
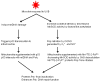
Manganese superoxide dismutase is a nuclear encoded primary antioxidant enzyme localized exclusively in the mitochondrial matrix. Genotoxic agents, such as ultraviolet (UV) radiation, generates oxidative stress and cause mitochondrial DNA (mtDNA) damage. The mtDNA polymerase (Polγ), a major constituent of nucleoids, is responsible for the replication and repair of the mitochondrial genome. Recent studies suggest that the mitochondria contain fidelity proteins and MnSOD constitutes an integral part of the nucleoid complex. However, it is not known whether or how MnSOD participates in the mitochondrial repair processes. Using skin tissue from C57BL/6 mice exposed to UVB radiation, we demonstrate that MnSOD has a critical role in preventing mtDNA damage by protecting the function of Polγ. Quantitative-PCR analysis shows an increase in mtDNA damage after UVB exposure. Immunofluorescence and immunoblotting studies demonstrate p53 translocation to the mitochondria and interaction with Polγ after UVB exposure. The mtDNA immunoprecipitation assay with Polγ and p53 antibodies in p53(+/+) and p53(-/-) mice demonstrates an interaction between MnSOD, p53 and Polγ. The results suggest that these proteins form a complex for the repair of UVB-associated mtDNA damage. The data also demonstrate that UVB exposure injures the mtDNA D-loop in a p53-dependent manner. Using MnSOD-deficient mice we demonstrate that UVB-induced mtDNA damage is MnSOD dependent. Exposure to UVB results in nitration and inactivation of Polγ, which is prevented by addition of the MnSOD mimetic Mn(III)TE-2-PyP(5+). These results demonstrate for the first time that MnSOD is a fidelity protein that maintains the activity of Polγ by preventing UVB-induced nitration and inactivation of Polγ. The data also demonstrate that MnSOD has a role along with p53 to prevent mtDNA damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
UVB-induced inactivation of manganese-containing superoxide dismutase promotes mitophagy via ROS-mediated mTORC2 pathway activation.J Biol Chem. 2019 Apr 26;294(17):6831-6842. doi: 10.1074/jbc.RA118.006595. Epub 2019 Mar 11. J Biol Chem. 2019. PMID: 30858178 Free PMC article.
-
Hepatic mitochondrial DNA depletion after an alcohol binge in mice: probable role of peroxynitrite and modulation by manganese superoxide dismutase.J Pharmacol Exp Ther. 2010 Mar;332(3):886-97. doi: 10.1124/jpet.109.160879. Epub 2009 Dec 16. J Pharmacol Exp Ther. 2010. PMID: 20016022
-
Sirt3-MnSOD axis represses nicotine-induced mitochondrial oxidative stress and mtDNA damage in osteoblasts.Acta Biochim Biophys Sin (Shanghai). 2015 Apr;47(4):306-12. doi: 10.1093/abbs/gmv013. Epub 2015 Mar 10. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25757953
-
MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx.Antioxid Redox Signal. 2014 Apr 1;20(10):1599-617. doi: 10.1089/ars.2013.5305. Epub 2013 Jun 8. Antioxid Redox Signal. 2014. PMID: 23581847 Free PMC article. Review.
-
Manganese superoxide dismutase: beyond life and death.Amino Acids. 2012 Jan;42(1):139-58. doi: 10.1007/s00726-010-0600-9. Epub 2010 May 8. Amino Acids. 2012. PMID: 20454814 Free PMC article. Review.
Cited by
-
Antioxidant gene therapy against neuronal cell death.Pharmacol Ther. 2014 May;142(2):206-30. doi: 10.1016/j.pharmthera.2013.12.007. Epub 2013 Dec 12. Pharmacol Ther. 2014. PMID: 24333264 Free PMC article. Review.
-
Mitochondrial Genome-Encoded Long Noncoding RNA and Mitochondrial Stability in Diabetic Retinopathy.Diabetes. 2023 Apr 1;72(4):520-531. doi: 10.2337/db22-0744. Diabetes. 2023. PMID: 36563021 Free PMC article.
-
Curbing cancer's sweet tooth: is there a role for MnSOD in regulation of the Warburg effect?Mitochondrion. 2013 May;13(3):170-88. doi: 10.1016/j.mito.2012.07.104. Epub 2012 Jul 20. Mitochondrion. 2013. PMID: 22820117 Free PMC article. Review.
-
Mitochondrial translocation of p53 modulates neuronal fate by preventing differentiation-induced mitochondrial stress.Antioxid Redox Signal. 2014 Sep 1;21(7):1009-24. doi: 10.1089/ars.2013.5417. Epub 2014 Mar 12. Antioxid Redox Signal. 2014. PMID: 24329038 Free PMC article.
-
Manganese superoxide dismutase, MnSOD and its mimics.Biochim Biophys Acta. 2012 May;1822(5):794-814. doi: 10.1016/j.bbadis.2011.12.002. Epub 2011 Dec 9. Biochim Biophys Acta. 2012. PMID: 22198225 Free PMC article. Review.
References
-
- Aitken GR, Henderson JR, Chang SC, McNeil CJ, Birch-Machin MA. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin Exp Dermatol. 2007;32:722–727. - PubMed
-
- Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. - PubMed
-
- Bakhanashvili M, Grinberg S, Bonda E, Simon AJ, Moshitch-Moshkovitz S, Rahav G. p53 in mitochondria enhances the accuracy of DNA synthesis. Cell Death Differ. 2008;15:1865–1874. - PubMed
-
- Batinic-Haberle I, Spasojevic I, Hambright P, Benov L, Crumbliss AL, Fridovich I. Relationship among Redox Potentials, Proton Dissociation Constants of Pyrrolic Nitrogens, and in Vivo and in Vitro Superoxide Dismutating Activities of Manganese(III) and Iron(III) Water-Soluble Porphyrins. Inorg Chem. 1999;38:4011–4022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

