Loss of breast epithelial marker hCLCA2 promotes epithelial-to-mesenchymal transition and indicates higher risk of metastasis
- PMID: 21909135
- PMCID: PMC4154589
- DOI: 10.1038/onc.2011.392
Loss of breast epithelial marker hCLCA2 promotes epithelial-to-mesenchymal transition and indicates higher risk of metastasis
Abstract
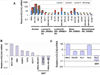
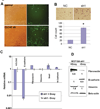
Transition between epithelial and mesenchymal states is a feature of both normal development and tumor progression. We report that expression of chloride channel accessory protein hCLCA2 is a characteristic of epithelial differentiation in the immortalized MCF10A and HMLE models, while induction of epithelial-to-mesenchymal transition by cell dilution, TGFβ or mesenchymal transcription factors sharply reduces hCLCA2 levels. Attenuation of hCLCA2 expression by lentiviral small hairpin RNA caused cell overgrowth and focus formation, enhanced migration and invasion, and increased mammosphere formation in methylcellulose. These changes were accompanied by downregulation of E-cadherin and upregulation of mesenchymal markers such as vimentin and fibronectin. Moreover, hCLCA2 expression is greatly downregulated in breast cancer cells with a mesenchymal or claudin-low profile. These observations suggest that loss of hCLCA2 may promote metastasis. We find that higher-than-median expression of hCLCA2 is associated with a one-third lower rate of metastasis over an 18-year period among breast cancer patients compared with lower-than-median (n=344, unfiltered for subtype). Thus, hCLCA2 is required for epithelial differentiation, and its loss during tumor progression contributes to metastasis. Overexpression of hCLCA2 has been reported to inhibit cell proliferation and is accompanied by increases in chloride current at the plasma membrane and reduced intracellular pH (pHi). We found that knockdown cells have sharply reduced chloride current and higher pHi, both characteristics of tumor cells. These results suggest a mechanism for the effects on differentiation. Loss of hCLCA2 may allow escape from pHi homeostatic mechanisms, permitting the higher intracellular and lower extracellular pH that are characteristic of aggressive tumor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Loss of CLCA4 promotes epithelial-to-mesenchymal transition in breast cancer cells.PLoS One. 2013 Dec 26;8(12):e83943. doi: 10.1371/journal.pone.0083943. eCollection 2013. PLoS One. 2013. PMID: 24386311 Free PMC article.
-
hCLCA2 Is a p53-Inducible Inhibitor of Breast Cancer Cell Proliferation.Cancer Res. 2009 Aug 15;69(16):6624-32. doi: 10.1158/0008-5472.CAN-08-4101. Epub 2009 Aug 4. Cancer Res. 2009. PMID: 19654313 Free PMC article.
-
Re-expression of detachment-inducible chloride channel mCLCA5 suppresses growth of metastatic breast cancer cells.J Biol Chem. 2004 Oct 1;279(40):41634-41. doi: 10.1074/jbc.M408334200. Epub 2004 Jul 30. J Biol Chem. 2004. PMID: 15292178
-
MicroRNAs regulate the epithelial-mesenchymal transition and influence breast cancer invasion and metastasis.Tumour Biol. 2017 Feb;39(2):1010428317691682. doi: 10.1177/1010428317691682. Tumour Biol. 2017. PMID: 28222665 Review.
-
Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors.Ups J Med Sci. 2012 May;117(2):217-24. doi: 10.3109/03009734.2012.659294. Epub 2012 Feb 29. Ups J Med Sci. 2012. PMID: 22376239 Free PMC article. Review.
Cited by
-
Downregulation of CLCA4 expression is associated with the development and progression of colorectal cancer.Oncol Lett. 2020 Jul;20(1):631-638. doi: 10.3892/ol.2020.11640. Epub 2020 May 18. Oncol Lett. 2020. PMID: 32565987 Free PMC article.
-
A pseudogene-signature in glioma predicts survival.J Exp Clin Cancer Res. 2015 Mar 4;34(1):23. doi: 10.1186/s13046-015-0137-6. J Exp Clin Cancer Res. 2015. PMID: 25880120 Free PMC article.
-
The calcium-activated chloride channel-associated protein rCLCA2 is expressed throughout rat epidermis, facilitates apoptosis and is downmodulated by UVB.Histochem Cell Biol. 2021 May;155(5):605-615. doi: 10.1007/s00418-021-01962-5. Epub 2021 Jan 23. Histochem Cell Biol. 2021. PMID: 33486586 Free PMC article.
-
Keratin 14-high subpopulation mediates lung cancer metastasis potentially through Gkn1 upregulation.Oncogene. 2019 Sep;38(36):6354-6369. doi: 10.1038/s41388-019-0889-0. Epub 2019 Jul 18. Oncogene. 2019. PMID: 31320708
-
Loss of CLCA4 promotes epithelial-to-mesenchymal transition in breast cancer cells.PLoS One. 2013 Dec 26;8(12):e83943. doi: 10.1371/journal.pone.0083943. eCollection 2013. PLoS One. 2013. PMID: 24386311 Free PMC article.
References
-
- Antonsson B, Conti F, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277(5324):370–372. - PubMed
-
- Barriere H, Poujeol C, et al. CFTR modulates programmed cell death by decreasing intracellular pH in Chinese hamster lung fibroblasts. Am. J. Physiol Cell Physiol. 2001;281(3):C810–C824. - PubMed
-
- Barry MA, Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem. Biophys. Res. Commun. 1992;186(2):782–789. - PubMed
-
- Beckley JR, Pauli BU, et al. Re-expression of detachment-inducible chloride channel mCLCA5 suppresses growth of metastatic breast cancer cells. J. Biol. Chem. 2004;279(40):41634–41641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

