Cellular and network mechanisms of genetically-determined absence seizures
- PMID: 21909233
- PMCID: PMC3168114
- DOI: 10.1017/S1472928807000209
Cellular and network mechanisms of genetically-determined absence seizures
Abstract
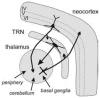
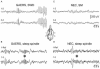
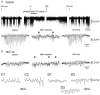
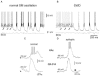
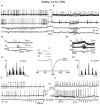
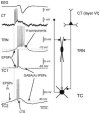
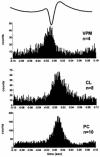
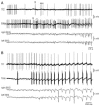
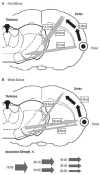
The absence epilepsies are characterized by recurrent episodes of loss of consciousness associated with generalized spike-and-wave discharges, with an abrupt onset and offset, in the thalamocortical system. In the absence of detailed neurophysiological studies in humans, many of the concepts regarding the pathophysiological basis of absence seizures are based on studies in animal models. Each of these models has its particular strengths and limitations, and the validity of findings from these models for the human condition cannot be assumed. Consequently, studies in different models have produced some conflicting findings and conclusions. A long-standing concept, based primarily from studies in vivo in cats and in vitro brain slices, is that these paroxysmal electrical events develop suddenly from sleep-related spindle oscillations. More specifically, it is proposed that the initial mechanisms that underlie absence-related spike-and-wave discharges are located in the thalamus, involving especially the thalamic reticular nucleus. By contrast, more recent studies in well-established, genetic models of absence epilepsy in rats demonstrate that spike-and-wave discharges originate in a cortical focus and develop from a wake-related natural corticothalamic sensorimotor rhythm. In this review we integrate recent findings showing that, in both the thalamus and the neocortex, genetically-determined, absence-related spike-and-wave discharges are the manifestation of hypersynchronized, cellular, rhythmic excitations and inhibitions that result from a combination of complex, intrinsic, synaptic mechanisms. Arguments are put forward supporting the hypothesis that layer VI corticothalamic neurons act as 'drivers' in the generation of spike-and-wave discharges in the somatosensory thalamocortical system that result in corticothalamic resonances particularly initially involving the thalamic reticular nucleus. However an important unresolved question is: what are the cellular and network mechanisms responsible for the switch from physiological, wake-related, natural oscillations into pathological spike-and-wave discharges? We speculate on possible answers to this, building particularly on recent findings from genetic models in rats.
Figures





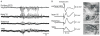
References
-
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the osterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1951–1922. - PubMed
-
- Avoli M, Gloor P. Role of the thalamus in generalized penicillin epilepsy: observations on decorticated cats. Experimental Neurology. 1982;77:386–402. - PubMed
-
- Avoli M, Gloor P, Kostopoulos G, Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. Journal of Neurophysiology. 1983;50:819–837. - PubMed
-
- Avoli M, Rogawski MA, Avanzini G. Generalized epileptic disorders: an update. Epilepsia. 2001;42:445–457. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
