Th2-polarised PrP-specific transgenic T-cells confer partial protection against murine scrapie
- PMID: 21909267
- PMCID: PMC3164648
- DOI: 10.1371/journal.ppat.1002216
Th2-polarised PrP-specific transgenic T-cells confer partial protection against murine scrapie
Abstract
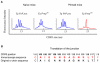
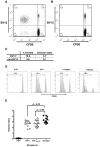
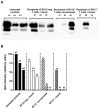
Several hurdles must be overcome in order to achieve efficient and safe immunotherapy against conformational neurodegenerative diseases. In prion diseases, the main difficulty is that the prion protein is tolerated as a self protein, which prevents powerful immune responses. Passive antibody therapy is effective only during early, asymptomatic disease, well before diagnosis is made. If efficient immunotherapy of prion diseases is to be achieved, it is crucial to understand precisely how immune tolerance against the prion protein can be overcome and which effector pathways may delay disease progression. To this end, we generated a transgenic mouse that expresses the ß-chain of a T cell receptor recognizing a PrP epitope presented by the class II major histocompatibility complex. The fact that the constraint is applied to only one TCR chain allows adaptation of the other chain according to the presence or absence of tolerogenic PrP. We first show that transgene-bearing T cells, pairing with rearranged α-chains conferring anti-PrP specificity, are systematically eliminated during ontogeny in PrP+ mice, suggesting that precursors with good functional avidity are rare in a normal individual. Second, we show that transgene-bearing T cells with anti-PrP specificity are not suppressed when transferred into PrP+ recipients and proliferate more extensively in a prion-infected host. Finally, such T cells provide protection through a cell-mediated pathway involving IL-4 production. These findings support the idea that cell-mediated immunity in neurodegenerative conditions may not be necessarily detrimental and may even contribute, when properly controlled, to the resolution of pathological processes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


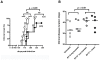

Similar articles
-
Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies.Science. 2001 Oct 5;294(5540):178-82. doi: 10.1126/science.1063093. Epub 2001 Sep 6. Science. 2001. PMID: 11546838
-
Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies.Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10608-12. doi: 10.1073/pnas.90.22.10608. Proc Natl Acad Sci U S A. 1993. PMID: 7902565 Free PMC article.
-
Mouse vaccination with dendritic cells loaded with prion protein peptides overcomes tolerance and delays scrapie.J Gen Virol. 2010 Mar;91(Pt 3):809-20. doi: 10.1099/vir.0.013417-0. Epub 2009 Oct 28. J Gen Virol. 2010. PMID: 19864503
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle.Dev Biol Stand. 1991;75:55-74. Dev Biol Stand. 1991. PMID: 1686599 Review.
Cited by
-
Exacerbation of experimental autoimmune encephalomyelitis in prion protein (PrPc)-null mice: evidence for a critical role of the central nervous system.J Neuroinflammation. 2012 Jan 26;9:25. doi: 10.1186/1742-2094-9-25. J Neuroinflammation. 2012. PMID: 22281016 Free PMC article.
-
Vaccines for prion diseases: a realistic goal?Cell Tissue Res. 2023 Apr;392(1):367-392. doi: 10.1007/s00441-023-03749-7. Epub 2023 Feb 11. Cell Tissue Res. 2023. PMID: 36764940 Free PMC article. Review.
-
B cell-specific S1PR1 deficiency blocks prion dissemination between secondary lymphoid organs.J Immunol. 2012 May 15;188(10):5032-40. doi: 10.4049/jimmunol.1200349. Epub 2012 Apr 13. J Immunol. 2012. PMID: 22504650 Free PMC article.
-
Prion diseases: immunotargets and therapy.Immunotargets Ther. 2016 Jun 16;5:57-68. doi: 10.2147/ITT.S64795. eCollection 2016. Immunotargets Ther. 2016. PMID: 27529062 Free PMC article. Review.
-
Exploring immunotherapeutic strategies for neurodegenerative diseases: a focus on Huntington's disease and Prion diseases.Acta Pharmacol Sin. 2025 Jun;46(6):1511-1538. doi: 10.1038/s41401-024-01455-w. Epub 2025 Jan 31. Acta Pharmacol Sin. 2025. PMID: 39890942 Review.
References
-
- Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–1152. - PubMed
-
- Heppner FL, Musahl C, Arrighi I, Klein MA, Rulicke T, et al. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science. 2001;294:178–182. - PubMed
-
- White AR, Enever P, Tayebi M, Mushens R, Linehan J, et al. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature. 2003;422:80–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

