HTLV-1 propels thymic human T cell development in "human immune system" Rag2⁻/⁻ gamma c⁻/⁻ mice
- PMID: 21909275
- PMCID: PMC3164654
- DOI: 10.1371/journal.ppat.1002231
HTLV-1 propels thymic human T cell development in "human immune system" Rag2⁻/⁻ gamma c⁻/⁻ mice
Abstract
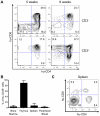
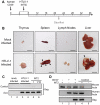
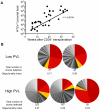
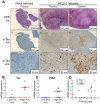
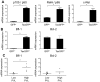
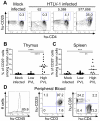
Alteration of early haematopoietic development is thought to be responsible for the onset of immature leukemias and lymphomas. We have previously demonstrated that Tax(HTLV-1) interferes with ß-selection, an important checkpoint of early thymopoiesis, indicating that human T-cell leukemia virus type 1 (HTLV-1) infection has the potential to perturb thymic human αβ T-cell development. To verify that inference and to clarify the impact of HTLV-1 infection on human T-cell development, we investigated the in vivo effects of HTLV-1 infection in a "Human Immune System" (HIS) Rag2⁻/⁻γ(c)⁻/⁻ mouse model. These mice were infected with HTLV-1, at a time when the three main subpopulations of human thymocytes have been detected. In all but two inoculated mice, the HTLV-1 provirus was found integrated in thymocytes; the proviral load increased with the length of the infection period. In the HTLV-1-infected mice we observed alterations in human T-cell development, the extent of which correlated with the proviral load. Thus, in the thymus of HTLV-1-infected HIS Rag2⁻/⁻γc⁻/⁻ mice, mature single-positive (SP) CD4⁺ and CD8⁺ cells were most numerous, at the expense of immature and double-positive (DP) thymocytes. These SP cells also accumulated in the spleen. Human lymphocytes from thymus and spleen were activated, as shown by the expression of CD25: this activation was correlated with the presence of tax mRNA and with increased expression of NF-kB dependent genes such as bfl-1, an anti-apoptotic gene, in thymocytes. Finally, hepato-splenomegaly, lymphadenopathy and lymphoma/thymoma, in which Tax was detected, were observed in HTLV-1-infected mice, several months after HTLV-1 infection. These results demonstrate the potential of the HIS Rag2⁻/⁻γ(c)⁻/⁻ animal model to elucidate the initial steps of the leukemogenic process induced by HTLV-1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Experimental acute adult T cell leukemia-lymphoma is associated with thymic atrophy in human T cell leukemia virus type I infection.Lab Invest. 1996 Mar;74(3):696-710. Lab Invest. 1996. PMID: 8600320
-
In vivo dynamics and adaptation of HTLV-1-infected clones under different clinical conditions.PLoS Pathog. 2021 Feb 1;17(2):e1009271. doi: 10.1371/journal.ppat.1009271. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33524072 Free PMC article.
-
De novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice.J Virol. 2006 Nov;80(21):10683-91. doi: 10.1128/JVI.01009-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943297 Free PMC article.
-
[Anti-tumor immunity in adult T-cell leukemia].Uirusu. 2004 Jun;54(1):67-74. doi: 10.2222/jsv.54.67. Uirusu. 2004. PMID: 15449906 Review. Japanese.
-
NF-κB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma.FEBS J. 2018 Sep;285(18):3324-3336. doi: 10.1111/febs.14492. Epub 2018 May 14. FEBS J. 2018. PMID: 29722927 Free PMC article. Review.
Cited by
-
Infection of the Ex Vivo Tonsil Model by HTLV-1 Envelope-Pseudotyped Viruses.Pathogens. 2023 Jan 24;12(2):182. doi: 10.3390/pathogens12020182. Pathogens. 2023. PMID: 36839454 Free PMC article.
-
Human T-cell Leukemia Virus Type 1 and Strongyloides stercoralis: Partners in Pathogenesis.Pathogens. 2020 Oct 29;9(11):904. doi: 10.3390/pathogens9110904. Pathogens. 2020. PMID: 33137906 Free PMC article. Review.
-
HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment.Viruses. 2017 Dec 21;10(1):1. doi: 10.3390/v10010001. Viruses. 2017. PMID: 29267225 Free PMC article. Review.
-
PDZ domain-binding motif of Tax sustains T-cell proliferation in HTLV-1-infected humanized mice.PLoS Pathog. 2018 Mar 22;14(3):e1006933. doi: 10.1371/journal.ppat.1006933. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29566098 Free PMC article.
-
HTLV-1 Infection and Pathogenesis: New Insights from Cellular and Animal Models.Int J Mol Sci. 2021 Jul 27;22(15):8001. doi: 10.3390/ijms22158001. Int J Mol Sci. 2021. PMID: 34360767 Free PMC article. Review.
References
-
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. - PubMed
-
- Nakano S, Ando Y, Saito K, Moriyama I, Ichijo M, et al. Primary infection of Japanese infants with adult T-cell leukaemia-associated retrovirus (ATLV): evidence for viral transmission from mothers to children. J Infect. 1986;12:205–212. - PubMed
-
- Aifantis I, Raetz E, Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol. 2008;8:380–390. - PubMed

