The general transcriptional repressor Tup1 is required for dimorphism and virulence in a fungal plant pathogen
- PMID: 21909277
- PMCID: PMC3164652
- DOI: 10.1371/journal.ppat.1002235
The general transcriptional repressor Tup1 is required for dimorphism and virulence in a fungal plant pathogen
Abstract
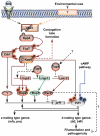
A critical step in the life cycle of many fungal pathogens is the transition between yeast-like growth and the formation of filamentous structures, a process known as dimorphism. This morphological shift, typically triggered by multiple environmental signals, is tightly controlled by complex genetic pathways to ensure successful pathogenic development. In animal pathogenic fungi, one of the best known regulators of dimorphism is the general transcriptional repressor, Tup1. However, the role of Tup1 in fungal dimorphism is completely unknown in plant pathogens. Here we show that Tup1 plays a key role in orchestrating the yeast to hypha transition in the maize pathogen Ustilago maydis. Deletion of the tup1 gene causes a drastic reduction in the mating and filamentation capacity of the fungus, in turn leading to a reduced virulence phenotype. In U. maydis, these processes are controlled by the a and b mating-type loci, whose expression depends on the Prf1 transcription factor. Interestingly, Δtup1 strains show a critical reduction in the expression of prf1 and that of Prf1 target genes at both loci. Moreover, we observed that Tup1 appears to regulate Prf1 activity by controlling the expression of the prf1 transcriptional activators, rop1 and hap2. Additionally, we describe a putative novel prf1 repressor, named Pac2, which seems to be an important target of Tup1 in the control of dimorphism and virulence. Furthermore, we show that Tup1 is required for full pathogenic development since tup1 deletion mutants are unable to complete the sexual cycle. Our findings establish Tup1 as a key factor coordinating dimorphism in the phytopathogen U. maydis and support a conserved role for Tup1 in the control of hypha-specific genes among animal and plant fungal pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Deletion of the Ustilago maydis ortholog of the Aspergillus sporulation regulator medA affects mating and virulence through pheromone response.Fungal Genet Biol. 2012 Jun;49(6):426-32. doi: 10.1016/j.fgb.2012.04.002. Epub 2012 Apr 19. Fungal Genet Biol. 2012. PMID: 22537792
-
UmTco1, a Hybrid Histidine Kinase Gene, Is Essential for the Sexual Development and Virulence of Ustilago maydis.J Microbiol Biotechnol. 2017 May 28;27(5):1010-1022. doi: 10.4014/jmb.1702.02001. J Microbiol Biotechnol. 2017. PMID: 28237997
-
The high-mobility-group domain transcription factor Rop1 is a direct regulator of prf1 in Ustilago maydis.Eukaryot Cell. 2005 Feb;4(2):379-91. doi: 10.1128/EC.4.2.379-391.2005. Eukaryot Cell. 2005. PMID: 15701800 Free PMC article.
-
Control of mating and development in Ustilago maydis.Antonie Van Leeuwenhoek. 1994;65(3):191-7. doi: 10.1007/BF00871946. Antonie Van Leeuwenhoek. 1994. PMID: 7847885 Review.
-
Dimorphism in fungal pathogens: Candida albicans and Ustilago maydis--similar inputs, different outputs.Curr Opin Microbiol. 2001 Apr;4(2):214-21. doi: 10.1016/s1369-5274(00)00191-0. Curr Opin Microbiol. 2001. PMID: 11282479 Review.
Cited by
-
The Coprinopsis cinerea Tup1 homologue Cag1 is required for gill formation during fruiting body morphogenesis.Biol Open. 2016 Dec 15;5(12):1844-1852. doi: 10.1242/bio.021246. Biol Open. 2016. PMID: 27815245 Free PMC article.
-
Investigating the Smuts: Common Cues, Signaling Pathways, and the Role of MAT in Dimorphic Switching and Pathogenesis.J Fungi (Basel). 2020 Dec 16;6(4):368. doi: 10.3390/jof6040368. J Fungi (Basel). 2020. PMID: 33339287 Free PMC article. Review.
-
The molecular mechanism of sporocyteless/nozzle in controlling Arabidopsis ovule development.Cell Res. 2015 Jan;25(1):121-34. doi: 10.1038/cr.2014.145. Epub 2014 Nov 7. Cell Res. 2015. PMID: 25378179 Free PMC article.
-
The Hos2 Histone Deacetylase Controls Ustilago maydis Virulence through Direct Regulation of Mating-Type Genes.PLoS Pathog. 2015 Aug 28;11(8):e1005134. doi: 10.1371/journal.ppat.1005134. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26317403 Free PMC article.
-
Strategies for Wheat Stripe Rust Pathogenicity Identified by Transcriptome Sequencing.PLoS One. 2013 Jun 26;8(6):e67150. doi: 10.1371/journal.pone.0067150. Print 2013. PLoS One. 2013. PMID: 23840606 Free PMC article.
References
-
- Schulz B, Banuett F, Dahl M, Schlesinger R, Schafer W, et al. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell. 1990;60:295–306. - PubMed
-
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. - PubMed
-
- Liu H. Co-regulation of pathogenesis with dimorphism and phenotypic switching in candida albicans, a commensal and a pathogen. Int J Med Microbiol. 2002;292:299–311. - PubMed
-
- Lin X. Cryptococcus neoformans: Morphogenesis, infection, and evolution. Infect Genet Evol. 2009;9:401–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

