Inhibition of competence development, horizontal gene transfer and virulence in Streptococcus pneumoniae by a modified competence stimulating peptide
- PMID: 21909280
- PMCID: PMC3164649
- DOI: 10.1371/journal.ppat.1002241
Inhibition of competence development, horizontal gene transfer and virulence in Streptococcus pneumoniae by a modified competence stimulating peptide
Abstract
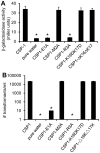
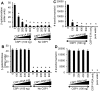
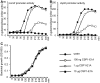
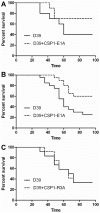
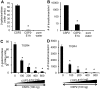
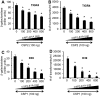
Competence stimulating peptide (CSP) is a 17-amino acid peptide pheromone secreted by Streptococcus pneumoniae. Upon binding of CSP to its membrane-associated receptor kinase ComD, a cascade of signaling events is initiated, leading to activation of the competence regulon by the response regulator ComE. Genes encoding proteins that are involved in DNA uptake and transformation, as well as virulence, are upregulated. Previous studies have shown that disruption of key components in the competence regulon inhibits DNA transformation and attenuates virulence. Thus, synthetic analogues that competitively inhibit CSPs may serve as attractive drugs to control pneumococcal infection and to reduce horizontal gene transfer during infection. We performed amino acid substitutions on conserved amino acid residues of CSP1 in an effort to disable DNA transformation and to attenuate the virulence of S. pneumoniae. One of the mutated peptides, CSP1-E1A, inhibited development of competence in DNA transformation by outcompeting CSP1 in time and concentration-dependent manners. CSP1-E1A reduced the expression of pneumococcal virulence factors choline binding protein D (CbpD) and autolysin A (LytA) in vitro, and significantly reduced mouse mortality after lung infection. Furthermore, CSP1-E1A attenuated the acquisition of an antibiotic resistance gene and a capsule gene in vivo. Finally, we demonstrated that the strategy of using a peptide inhibitor is applicable to other CSP subtype, including CSP2. CSP1-E1A and CSP2-E1A were able to cross inhibit the induction of competence and DNA transformation in pneumococcal strains with incompatible ComD subtypes. These results demonstrate the applicability of generating competitive analogues of CSPs as drugs to control horizontal transfer of antibiotic resistance and virulence genes, and to attenuate virulence during infection by S. pneumoniae.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

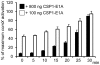

References
-
- Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. 2010;16:411–418. - PubMed
-
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. - PubMed
-
- Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, et al. Diagnosis, Microbial Epidemiology, and Antibiotic Treatment of Acute Otitis Media in Children: A Systematic Review. JAMA. 2010;304:2161–2169. - PubMed
-
- Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010;16:217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

