The acute HIV infection: implications for intervention, prevention and development of an effective AIDS vaccine
- PMID: 21909345
- PMCID: PMC3168513
- DOI: 10.1016/j.coviro.2011.05.007
The acute HIV infection: implications for intervention, prevention and development of an effective AIDS vaccine
Abstract
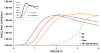
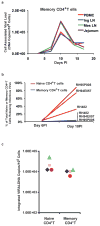
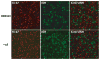
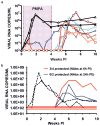
Effective preventive measures against HIV must function near the time of virus transmission to prevent the establishment of a chronic infection. Low-dose SIV/SHIV infections by multiple routes lead to remarkably rapid systemic dissemination of virus and large numbers of infected cells during the initial weeks of the acute infection. Here we describe the narrow time-frame during which potent post-exposure interventions such as anti-retroviral therapy or the administration of high-titered neutralizing antibodies can block the establishment of the in vivo infection. This short window of opportunity is applicable to HIV infections and represents a formidable challenge for developing effective chemoprophylaxis and vaccine approaches.
Figures
Similar articles
-
Vaccine-induced V1V2-specific antibodies control and or protect against infection with HIV, SIV and SHIV.Curr Opin HIV AIDS. 2019 Jul;14(4):309-317. doi: 10.1097/COH.0000000000000551. Curr Opin HIV AIDS. 2019. PMID: 30994501 Free PMC article. Review.
-
Protection against chronic infection and AIDS by an HIV envelope peptide-cocktail vaccine in a pathogenic SHIV-rhesus model.Vaccine. 2001 Dec 12;20(5-6):813-25. doi: 10.1016/s0264-410x(01)00408-x. Vaccine. 2001. PMID: 11738745
-
Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine.J Virol. 2012 Feb;86(4):2239-50. doi: 10.1128/JVI.06175-11. Epub 2011 Dec 7. J Virol. 2012. PMID: 22156519 Free PMC article. Clinical Trial.
-
Vaccination with the Conserved Caveolin-1 Binding Motif in Human Immunodeficiency Virus Type 1 Glycoprotein gp41 Delays the Onset of Viral Infection and Provides Partial Protection in Simian/Human Immunodeficiency Virus-Challenged Cynomolgus Macaques.J Virol. 2018 Aug 29;92(18):e00370-18. doi: 10.1128/JVI.00370-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976675 Free PMC article.
-
Impact of MHC class I diversity on immune control of immunodeficiency virus replication.Nat Rev Immunol. 2008 Aug;8(8):619-30. doi: 10.1038/nri2357. Nat Rev Immunol. 2008. PMID: 18617886 Free PMC article. Review.
Cited by
-
Contribution of HIV-1 genomes that do not integrate to the basic reproductive ratio of the virus.J Theor Biol. 2015 Feb 21;367:222-229. doi: 10.1016/j.jtbi.2014.12.004. Epub 2014 Dec 12. J Theor Biol. 2015. PMID: 25496730 Free PMC article.
-
Neutralizing antibody affords comparable protection against vaginal and rectal simian/human immunodeficiency virus challenge in macaques.AIDS. 2016 Jun 19;30(10):1543-51. doi: 10.1097/QAD.0000000000001102. AIDS. 2016. PMID: 27243773 Free PMC article.
-
Lack of viral control and development of combination antiretroviral therapy escape mutations in macaques after bone marrow transplantation.AIDS. 2015 Aug 24;29(13):1597-606. doi: 10.1097/QAD.0000000000000702. AIDS. 2015. PMID: 26372270 Free PMC article.
-
Using nonhuman primates to model HIV transmission.Curr Opin HIV AIDS. 2013 Jul;8(4):280-7. doi: 10.1097/COH.0b013e328361cfff. Curr Opin HIV AIDS. 2013. PMID: 23666391 Free PMC article. Review.
-
An HIV-1 replication pathway utilizing reverse transcription products that fail to integrate.J Virol. 2013 Dec;87(23):12701-20. doi: 10.1128/JVI.01939-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049167 Free PMC article.
References
-
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. This study describes the first successful anti-HIV microbicide to prevent virus transmission in women. - PMC - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Gasper-Smith N, Crossman DM, Whitesides JF, Mensali N, Ottinger JS, Plonk SG, Moody MA, Ferrari G, Weinhold KJ, Miller SE, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–7710. - PMC - PubMed
-
- Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. This report describes the establishment of SIV founder infections in the female genital tract following mucosal transmission of virus. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

