The K+ channel opener 1-EBIO potentiates residual function of mutant CFTR in rectal biopsies from cystic fibrosis patients
- PMID: 21909392
- PMCID: PMC3164200
- DOI: 10.1371/journal.pone.0024445
The K+ channel opener 1-EBIO potentiates residual function of mutant CFTR in rectal biopsies from cystic fibrosis patients
Abstract
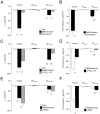
Background: The identification of strategies to improve mutant CFTR function remains a key priority in the development of new treatments for cystic fibrosis (CF). Previous studies demonstrated that the K⁺ channel opener 1-ethyl-2-benzimidazolone (1-EBIO) potentiates CFTR-mediated Cl⁻ secretion in cultured cells and mouse colon. However, the effects of 1-EBIO on wild-type and mutant CFTR function in native human colonic tissues remain unknown.
Methods: We studied the effects of 1-EBIO on CFTR-mediated Cl⁻ secretion in rectal biopsies from 47 CF patients carrying a wide spectrum of CFTR mutations and 57 age-matched controls. Rectal tissues were mounted in perfused micro-Ussing chambers and the effects of 1-EBIO were compared in control tissues, CF tissues expressing residual CFTR function and CF tissues with no detectable Cl⁻ secretion.
Results: Studies in control tissues demonstrate that 1-EBIO activated CFTR-mediated Cl⁻ secretion in the absence of cAMP-mediated stimulation and potentiated cAMP-induced Cl⁻ secretion by 39.2±6.7% (P<0.001) via activation of basolateral Ca²⁺-activated and clotrimazole-sensitive KCNN4 K⁺ channels. In CF specimens, 1-EBIO potentiated cAMP-induced Cl⁻ secretion in tissues with residual CFTR function by 44.4±11.5% (P<0.001), but had no effect on tissues lacking CFTR-mediated Cl⁻ conductance.
Conclusions: We conclude that 1-EBIO potentiates Cl⁻secretion in native CF tissues expressing CFTR mutants with residual Cl⁻ channel function by activation of basolateral KCNN4 K⁺ channels that increase the driving force for luminal Cl⁻ exit. This mechanism may augment effects of CFTR correctors and potentiators that increase the number and/or activity of mutant CFTR channels at the cell surface and suggests KCNN4 as a therapeutic target for CF.
Conflict of interest statement
Figures




Similar articles
-
Modulation of Ca2+-activated Cl- secretion by basolateral K+ channels in human normal and cystic fibrosis airway epithelia.Pediatr Res. 2003 Apr;53(4):608-18. doi: 10.1203/01.PDR.0000057204.51420.DC. Epub 2003 Feb 5. Pediatr Res. 2003. PMID: 12612194
-
Measurements of CFTR-mediated Cl- secretion in human rectal biopsies constitute a robust biomarker for Cystic Fibrosis diagnosis and prognosis.PLoS One. 2012;7(10):e47708. doi: 10.1371/journal.pone.0047708. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082198 Free PMC article.
-
Activation of Ca(2+)- and cAMP-sensitive K(+) channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone.Am J Physiol. 1999 Jul;277(1):C111-20. doi: 10.1152/ajpcell.1999.277.1.C111. Am J Physiol. 1999. PMID: 10409114
-
Role of CFTR in the colon.Annu Rev Physiol. 2000;62:467-91. doi: 10.1146/annurev.physiol.62.1.467. Annu Rev Physiol. 2000. PMID: 10845099 Review.
-
CFTR: a hub for kinases and crosstalk of cAMP and Ca2+.FEBS J. 2013 Sep;280(18):4417-29. doi: 10.1111/febs.12457. Epub 2013 Aug 27. FEBS J. 2013. PMID: 23895508 Review.
Cited by
-
Potential of Intestinal Current Measurement for Personalized Treatment of Patients with Cystic Fibrosis.J Pers Med. 2021 May 8;11(5):384. doi: 10.3390/jpm11050384. J Pers Med. 2021. PMID: 34066648 Free PMC article. Review.
-
Homozygous CFTR mutation M348K in a boy with respiratory symptoms and failure to thrive. Disease-causing mutation or benign alteration?Eur J Pediatr. 2012 Jul;171(7):1039-46. doi: 10.1007/s00431-012-1672-1. Epub 2012 Jan 25. Eur J Pediatr. 2012. PMID: 22274833
-
Investigating CFTR and KCa3.1 Protein/Protein Interactions.PLoS One. 2016 Apr 19;11(4):e0153665. doi: 10.1371/journal.pone.0153665. eCollection 2016. PLoS One. 2016. PMID: 27092946 Free PMC article.
-
CFTR regulates early pathogenesis of chronic obstructive lung disease in βENaC-overexpressing mice.PLoS One. 2012;7(8):e44059. doi: 10.1371/journal.pone.0044059. Epub 2012 Aug 24. PLoS One. 2012. PMID: 22937152 Free PMC article.
-
Functional analysis of the p.[Arg74Trp;Val201Met;Asp1270Asn]/p.Phe508del CFTR mutation genotype in human native colon.Mol Genet Genomic Med. 2019 Feb;7(2):e00526. doi: 10.1002/mgg3.526. Epub 2019 Jan 1. Mol Genet Genomic Med. 2019. PMID: 30600599 Free PMC article.
References
-
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. - PubMed
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 5121–5188.
-
- Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. - PubMed
-
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. - PubMed
-
- Hamosh A, Trapnell BC, Zeitlin PL, Montrose-Rafizadeh C, Rosenstein BJ, et al. Severe deficiency of cystic fibrosis transmembrane conductance regulator messenger RNA carrying nonsense mutations R553X and W1316X in respiratory epithelial cells of patients with cystic fibrosis. J Clin Invest. 1991;88:1880–1885. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

