Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR
- PMID: 21909403
- PMCID: PMC3164657
- DOI: 10.1371/journal.pone.0023637
Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR
Abstract
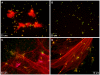
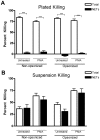
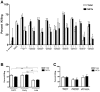
The inability of neutrophils to eradicate Pseudomonas aeruginosa within the cystic fibrosis (CF) airway eventually results in chronic infection by the bacteria in nearly 80 percent of patients. Phagocytic killing of P. aeruginosa by CF neutrophils is impaired due to decreased cystic fibrosis transmembrane conductance regulator (CFTR) function and virulence factors acquired by the bacteria. Recently, neutrophil extracellular traps (NETs), extracellular structures composed of neutrophil chromatin complexed with granule contents, were identified as an alternative mechanism of pathogen killing. The hypothesis that NET-mediated killing of P. aeruginosa is impaired in the context of the CF airway was tested. P. aeruginosa induced NET formation by neutrophils from healthy donors in a bacterial density dependent fashion. When maintained in suspension through continuous rotation, P. aeruginosa became physically associated with NETs. Under these conditions, NETs were the predominant mechanism of killing, across a wide range of bacterial densities. Peripheral blood neutrophils isolated from CF patients demonstrated no impairment in NET formation or function against P. aeruginosa. However, isogenic clinical isolates of P. aeruginosa obtained from CF patients early and later in the course of infection demonstrated an acquired capacity to withstand NET-mediated killing in 8 of 9 isolates tested. This resistance correlated with development of the mucoid phenotype, but was not a direct result of the excess alginate production that is characteristic of mucoidy. Together, these results demonstrate that neutrophils can kill P. aeruginosa via NETs, and in vitro this response is most effective under non-stationary conditions with a low ratio of bacteria to neutrophils. NET-mediated killing is independent of CFTR function or bacterial opsonization. Failure of this response in the context of the CF airway may occur, in part, due to an acquired resistance against NET-mediated killing by CF strains of P. aeruginosa.
Conflict of interest statement
Figures



Similar articles
-
NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes.Immunol Lett. 2014 Aug;160(2):186-94. doi: 10.1016/j.imlet.2014.03.003. Epub 2014 Mar 23. Immunol Lett. 2014. PMID: 24670966
-
Reactive-oxygen-species-mediated P. aeruginosa killing is functional in human cystic fibrosis macrophages.PLoS One. 2013 Aug 19;8(8):e71717. doi: 10.1371/journal.pone.0071717. eCollection 2013. PLoS One. 2013. PMID: 23977124 Free PMC article.
-
Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation.J Immunol. 2014 May 15;192(10):4728-38. doi: 10.4049/jimmunol.1301589. Epub 2014 Apr 16. J Immunol. 2014. PMID: 24740504 Free PMC article. Clinical Trial.
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
-
Harnessing Neutrophil Survival Mechanisms during Chronic Infection by Pseudomonas aeruginosa: Novel Therapeutic Targets to Dampen Inflammation in Cystic Fibrosis.Front Cell Infect Microbiol. 2017 Jun 30;7:243. doi: 10.3389/fcimb.2017.00243. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713772 Free PMC article. Review.
Cited by
-
Mycobacterium abscessus induces a limited pattern of neutrophil activation that promotes pathogen survival.PLoS One. 2013;8(2):e57402. doi: 10.1371/journal.pone.0057402. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451220 Free PMC article.
-
Host-pathogen interplay in the respiratory environment of cystic fibrosis.J Cyst Fibros. 2015 Jul;14(4):431-439. doi: 10.1016/j.jcf.2015.02.008. Epub 2015 Mar 19. J Cyst Fibros. 2015. PMID: 25800687 Free PMC article. Review.
-
Understanding the Entanglement: Neutrophil Extracellular Traps (NETs) in Cystic Fibrosis.Front Cell Infect Microbiol. 2017 Apr 6;7:104. doi: 10.3389/fcimb.2017.00104. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28428948 Free PMC article. Review.
-
Neutrophil Extracellular Traps and Respiratory Disease.J Clin Med. 2024 Apr 19;13(8):2390. doi: 10.3390/jcm13082390. J Clin Med. 2024. PMID: 38673662 Free PMC article. Review.
-
Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase.PLoS One. 2013;8(1):e54205. doi: 10.1371/journal.pone.0054205. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23342104 Free PMC article.
References
-
- Comeau AM, Parad RB, Dorkin HL, Dovey M, Gerstle R, et al. Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: a cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatrics. 2004;113:1573–1581. - PubMed
-
- Sontag MK, Hammond KB, Zielenski J, Wagener JS, Accurso FJ. Two-tiered immunoreactive trypsinogen-based newborn screening for cystic fibrosis in Colorado: screening efficacy and diagnostic outcomes. J Pediatr. 2005;147:S83–88. - PubMed
-
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. - PubMed
-
- Cystic Fibrosis Foundation. Bethesda, Maryland; 2009. Cystic Fibrosis Foundation Patient Registry 2008 Annual Data Report.
-
- Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155:623–628 e621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

