Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid beta-oxidation in rats fed a methionine-choline deficient diet
- PMID: 21909411
- PMCID: PMC3164715
- DOI: 10.1371/journal.pone.0024084
Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid beta-oxidation in rats fed a methionine-choline deficient diet
Abstract
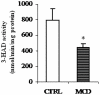
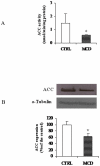
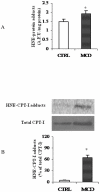
There is growing evidence that mitochondrial dysfunction, and more specifically fatty acid β-oxidation impairment, is involved in the pathophysiology of non-alcoholic steatohepatitis (NASH). The goal of the present study was to achieve more understanding on the modification/s of carnitinepalmitoyltransferase-I (CPT-I), the rate-limiting enzyme of the mitochondrial fatty acid β-oxidation, during steatohepatitis. A high fat/methionine-choline deficient (MCD) diet, administered for 4 weeks, was used to induce NASH in rats.We demonstrated that CPT-I activity decreased, to the same extent, both in isolated liver mitochondria and in digitonin-permeabilized hepatocytes from MCD-diet fed rats.At the same time, the rate of total fatty acid oxidation to CO(2) and ketone bodies, measured in isolated hepatocytes, was significantly lowered in treated animals when compared to controls. Finally, an increase in CPT-I mRNA abundance and protein content, together with a high level of CPT-I protein oxidation was observed in treated rats. A posttranslational modification of rat CPT-I during steatohepatitis has been here discussed.
Conflict of interest statement
Figures




References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. - PubMed
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. - PubMed
-
- Serviddio G, Bellanti F, Tamborra R, Rollo T, Romano AD, et al. Alterations of hepatic ATP homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. Eur J Clin Invest. 2008;38:245–252. - PubMed
-
- Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, et al. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

