Therapeutic enhancement of protective immunity during experimental leishmaniasis
- PMID: 21909452
- PMCID: PMC3167777
- DOI: 10.1371/journal.pntd.0001316
Therapeutic enhancement of protective immunity during experimental leishmaniasis
Abstract
Background: Leishmaniasis remains a significant cause of morbidity and mortality in the tropics. Available therapies are problematic due to toxicity, treatment duration and emerging drug resistance. Mouse models of leishmaniasis have demonstrated that disease outcome depends critically on the balance between effector and regulatory CD4(+) T cell responses, something mirrored in descriptive studies of human disease. Recombinant IL-2/diphtheria toxin fusion protein (rIL-2/DTx), a drug that is FDA-approved for the treatment of cutaneous T cell lymphoma, has been reported to deplete regulatory CD4(+) T cells.
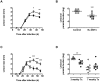
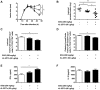
Methodology/principal findings: We investigated the potential efficacy of rIL-2/DTx as adjunctive therapy for experimental infection with Leishmania major. Treatment with rIL-2/DTx suppressed lesional regulatory T cell numbers and was associated with significantly increased antigen-specific IFN-γ production, enhanced lesion resolution and decreased parasite burden. Combined administration of rIL-2/DTx and sodium stibogluconate had additive biological and therapeutic effects, allowing for reduced duration or dose of sodium stibogluconate therapy.
Conclusions/significance: These data suggest that pharmacological suppression of immune counterregulation using a commercially available drug originally developed for cancer therapy may have practical therapeutic utility in leishmaniasis. Rational reinvestigation of the efficacy of drugs approved for other indications in experimental models of neglected tropical diseases has promise in providing new candidates to the drug discovery pipeline.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with sodium stibogluconate and gamma interferon depends on continued interleukin-12 production.Infect Immun. 1997 Aug;65(8):3225-30. doi: 10.1128/iai.65.8.3225-3230.1997. Infect Immun. 1997. PMID: 9234779 Free PMC article.
-
Activity of pentostam (sodium stibogluconate) against cutaneous leishmaniasis in mice treated with neutralizing anti-interferon-gamma antibody.Am J Trop Med Hyg. 1995 Jul;53(1):55-60. Am J Trop Med Hyg. 1995. PMID: 7625533
-
Synergistic Effect Of Oral Allopurinol And Intralesional Sodium Stibogluconate In The Treatment Of Cutaneous Leishmaniasis.J Ayub Med Coll Abbottabad. 2020 Oct-Dec;32(4):558-561. J Ayub Med Coll Abbottabad. 2020. PMID: 33225663 Clinical Trial.
-
Treatment of cutaneous leishmaniasis in travelers 2009.J Travel Med. 2009 Mar-Apr;16(2):123-31. doi: 10.1111/j.1708-8305.2008.00286.x. J Travel Med. 2009. PMID: 19335813 Review. No abstract available.
-
[Cutaneous leishmaniasis].Hautarzt. 2003 Jun;54(6):506-12. doi: 10.1007/s00105-003-0530-5. Epub 2003 Apr 18. Hautarzt. 2003. PMID: 12759734 Review. German.
Cited by
-
Regulation of Inflammation by IL-17A and IL-17F Modulates Non-Alcoholic Fatty Liver Disease Pathogenesis.PLoS One. 2016 Feb 19;11(2):e0149783. doi: 10.1371/journal.pone.0149783. eCollection 2016. PLoS One. 2016. PMID: 26895034 Free PMC article.
-
Suppressor Cell-Depleting Immunotherapy With Denileukin Diftitox is an Effective Host-Directed Therapy for Tuberculosis.J Infect Dis. 2017 Jun 15;215(12):1883-1887. doi: 10.1093/infdis/jix208. J Infect Dis. 2017. PMID: 28863467 Free PMC article.
-
Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice.Mol Metab. 2016 Sep 21;5(11):1121-1130. doi: 10.1016/j.molmet.2016.09.008. eCollection 2016 Nov. Mol Metab. 2016. PMID: 27818938 Free PMC article.
-
Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling.Nat Med. 2017 Jul;23(7):829-838. doi: 10.1038/nm.4346. Epub 2017 Jun 12. Nat Med. 2017. PMID: 28604704 Free PMC article.
-
Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: a review.Pathog Glob Health. 2016 Sep;110(6):247-260. doi: 10.1080/20477724.2016.1232042. Epub 2016 Sep 23. Pathog Glob Health. 2016. PMID: 27660895 Free PMC article. Review.
References
-
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. - PubMed
-
- Karp CL, Neva FA. Tropical infectious diseases in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;28:947–963; quiz 964–945. - PubMed
-
- Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

