Disturbed osteoblastic differentiation of fibrous hamartoma cell from congenital pseudarthrosis of the tibia associated with neurofibromatosis type I
- PMID: 21909471
- PMCID: PMC3162204
- DOI: 10.4055/cios.2011.3.3.230
Disturbed osteoblastic differentiation of fibrous hamartoma cell from congenital pseudarthrosis of the tibia associated with neurofibromatosis type I
Abstract
Background: Fibrous hamartoma is the key pathology of congenital pseudarthrosis of the tibia (CPT), which was shown to have low osteogenicity and high osteoclastogenicity. This study further investigated the mechanism of impaired osteoblastic differentiation of fibrous hamartoma cells.
Methods: Fibroblast-like cells were obtained from enzymatically dissociated fibrous hamartomas of 11 patients with CPT associated with neurofibromatosis type I (NF1). Periosteal cells were also obtained from the distal tibial periosteum of 3 patients without CPT or NF1 as control. The mRNA levels of Wnt ligands and their canonical receptors, such as Lrp5 and β-catenin, were assayed using reverse transcriptase PCR (RT-PCR). Changes in mRNA expression of osteoblast marker genes by rhBMP2 treatment were assayed using quantitative real time RT-PCR. Changes in mRNA expression of transcription factors specifically involved in osteoblastic differentiation by rhBMP2 treatment was also assayed using quantitative real-time RT-PCR.
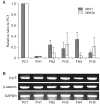
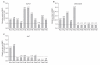
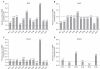
Results: Wnt1 and Wnt3a mRNA expression was lower in fibrous hamartoma than in tibial periosteal cells, but their canonical receptors did not show significant difference. Response of osteoblastic marker gene expression to rhBMP2 treatment showed patient-to-patient variability. Col1a1 mRNA expression was up-regulated in most fibrous hamartoma tissues, osteocalcin was up-regulated in a small number of patients, and ALP expression was down-regulated in most fibrous hamartoma tissues. Changes in mRNA expression of the transcription factors in response to rhBMP2 also showed factor-to-factor and patient-to-patient variability. Dlx5 was consistently up-regulated by rhBMP2 treatment in all fibrous hamartoma tissues tested. Msx2 expression was down-regulated by rhBMP2 in most cases but by lesser extent than control tissue. Runx2 expression was up-regulated in 8 out of 18 fibrous hamartoma tissues tested. Osterix expression was up-regulated in 2 and down-regulated in 3 fibrous hamartoma tissues.
Conclusions: Congenital pseudarthrosis of the tibia appears to be caused by fibrous hamartoma originating from aberrant growth of Nf1 haploinsufficient periosteal cells, which failed in terminal osteoblastic differentiation and arrested at a certain stage of this process. This pathomechanism of CPT should be targeted in the development of novel therapeutic biologic intervention.
Keywords: Congenital; Osteoblastic differentiation; Pseudarthrosis.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1.J Bone Joint Surg Am. 2008 Dec;90(12):2735-44. doi: 10.2106/JBJS.H.00014. J Bone Joint Surg Am. 2008. PMID: 19047720
-
Is double inactivation of the Nf1 gene responsible for the development of congenital pseudarthrosis of the tibia associated with NF1?J Orthop Res. 2012 Oct;30(10):1535-40. doi: 10.1002/jor.22121. Epub 2012 Apr 5. J Orthop Res. 2012. PMID: 22488919
-
A murine model of neurofibromatosis type 1 tibial pseudarthrosis featuring proliferative fibrous tissue and osteoclast-like cells.J Bone Miner Res. 2012 Jan;27(1):68-78. doi: 10.1002/jbmr.528. J Bone Miner Res. 2012. PMID: 21956219
-
Prevalence of neurofibromatosis type 1 in congenital pseudarthrosis of the tibia.Eur J Pediatr. 2016 Sep;175(9):1193-1198. doi: 10.1007/s00431-016-2757-z. Epub 2016 Aug 12. Eur J Pediatr. 2016. PMID: 27519821 Review.
-
Congenital Pseudarthrosis of the Tibia: A Comprehensive Literature Review.JBJS Rev. 2025 Jun 5;13(6). doi: 10.2106/JBJS.RVW.25.00035. eCollection 2025 Jun 1. JBJS Rev. 2025. PMID: 40472164 Review.
Cited by
-
Management of congenital pseudarthrosis of the tibia with the Ilizarov method in a paediatric population: influence of aetiological factors.Int Orthop. 2016 Feb;40(2):331-9. doi: 10.1007/s00264-015-3029-7. Epub 2015 Nov 7. Int Orthop. 2016. PMID: 26546064
-
Capturing the wide variety of impaired fracture healing phenotypes in Neurofibromatosis Type 1 with eight key factors: a computational study.Sci Rep. 2016 Jan 29;7:20010. doi: 10.1038/srep20010. Sci Rep. 2016. PMID: 26822862 Free PMC article.
-
Congenital pseudarthrosis of the tibia: Management and complications.Indian J Orthop. 2012 Nov;46(6):616-26. doi: 10.4103/0019-5413.104184. Indian J Orthop. 2012. PMID: 23325962 Free PMC article.
-
The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillofacial trauma.Chin J Traumatol. 2017 Feb;20(1):1-3. doi: 10.1016/j.cjtee.2016.05.004. Epub 2017 Feb 9. Chin J Traumatol. 2017. PMID: 28236566 Free PMC article. Review.
-
The reduced osteogenic potential of Nf1-deficient osteoprogenitors is EGFR-independent.Bone. 2018 Jan;106:103-111. doi: 10.1016/j.bone.2017.10.012. Epub 2017 Oct 12. Bone. 2018. PMID: 29032173 Free PMC article.
References
-
- Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000;9(1):3–10. - PubMed
-
- Hefti F, Bollini G, Dungl P, et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9(1):11–15. - PubMed
-
- Crawford AH, Schorry EK. Neurofibromatosis update. J Pediatr Orthop. 2006;26(3):413–423. - PubMed
-
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89(1):1–6. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

