Intrinsically disordered proteins as molecular shields
- PMID: 21909508
- PMCID: PMC5365143
- DOI: 10.1039/c1mb05263b
Intrinsically disordered proteins as molecular shields
Abstract
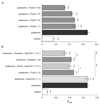
The broad family of LEA proteins are intrinsically disordered proteins (IDPs) with several potential roles in desiccation tolerance, or anhydrobiosis, one of which is to limit desiccation-induced aggregation of cellular proteins. We show here that this activity, termed molecular shield function, is distinct from that of a classical molecular chaperone, such as HSP70 - while HSP70 reduces aggregation of citrate synthase (CS) on heating, two LEA proteins, a nematode group 3 protein, AavLEA1, and a plant group 1 protein, Em, do not; conversely, the LEA proteins reduce CS aggregation on desiccation, while HSP70 lacks this ability. There are also differences in interaction with client proteins - HSP70 can be co-immunoprecipitated with a polyglutamine-containing client, consistent with tight complex formation, whereas the LEA proteins can not, although a loose interaction is observed by Förster resonance energy transfer. In a further exploration of molecular shield function, we demonstrate that synthetic polysaccharides, like LEA proteins, are able to reduce desiccation-induced aggregation of a water-soluble proteome, consistent with a steric interference model of anti-aggregation activity. If molecular shields operate by reducing intermolecular cohesion rates, they should not protect against intramolecular protein damage. This was tested using the monomeric red fluorescent protein, mCherry, which does not undergo aggregation on drying, but the absorbance and emission spectra of its intrinsic fluorophore are dramatically reduced, indicative of intramolecular conformational changes. As expected, these changes are not prevented by AavLEA1, except for a slight protection at high molar ratios, and an AavLEA1-mCherry fusion protein is damaged to the same extent as mCherry alone. A recent hypothesis proposed that proteomes from desiccation-tolerant species contain a higher degree of disorder than intolerant examples, and that this might provide greater intrinsic stability, but a bioinformatics survey does not support this, since there are no significant differences in the degree of disorder between desiccation tolerant and intolerant species. It seems clear therefore that molecular shield function is largely an intermolecular activity implemented by specialist IDPs, distinct from molecular chaperones, but with a role in proteostasis.
Figures
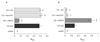




References
-
- Crowe JH, Hoekstra FA, Crowe LM. Annu Rev Physiol. 1992;54:579–599. - PubMed
-
- Burnell AM, Tunnacliffe A. In: Molecular and Physiological Basis of Nematode Survival. Perry RN, Wharton D, editors. CABI Publishing; Wallingford, UK: 2010.
-
- Clegg JS. Comp Biochem Physiol Part B: Biochem Mol Biol. 2001;128:613–624. - PubMed
-
- Alpert P. J Exp Biol. 2006;209:1575–1584. - PubMed
-
- Gadd GM, Chalmers K, Reed RH. FEMS Microbiol Lett. 1987;48:249–254.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

