Potentiation of brain stimulation reward by morphine: effects of neurokinin-1 receptor antagonism
- PMID: 21909635
- PMCID: PMC3484369
- DOI: 10.1007/s00213-011-2469-z
Potentiation of brain stimulation reward by morphine: effects of neurokinin-1 receptor antagonism
Abstract
Rationale: The abuse potential of opioids may be due to their reinforcing and rewarding effects, which may be attenuated by neurokinin-1 receptor (NK1R) antagonists.
Objective: This study was conducted to measure the effects of opioid and NK1R blockade on the potentiation of brain stimulation reward (BSR) by morphine using the intracranial self-stimulation method.
Methods: Adult male C57BL/6J mice (n = 15) were implanted with unipolar stimulating electrodes in the lateral hypothalamus and trained to respond for varying frequencies of rewarding electrical stimulation. The BSR threshold (θ(0)) and maximum response rate (MAX) were determined before and after intraperitoneal administration of saline, morphine (1.0-17.0 mg/kg), or the NK1R antagonists L-733,060 (1.0-17.0 mg/kg) and L-703,606 (1.0-17.0 mg/kg). In morphine antagonism experiments, naltrexone (0.1-1.0 mg/kg) or 10.0 mg/kg L-733,060 or L-703,606 was administered 15 min before morphine (1.0-10.0 mg/kg) or saline.
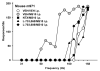
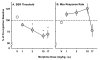
Results: Morphine dose-dependently decreased θ(0) (maximum effect = 62% of baseline) and altered MAX when compared to saline. L-703,606 and L-733,060 altered θ(0); 10.0 mg/kg L-733,060 and L-703,606, which did not affect θ(0) or MAX, attenuated the effects of 3.0 and 10.0 mg/kg morphine, and 1.0 and 0.3 mg/kg naltrexone blocked the effects of 10.0 mg/kg morphine. Naltrexone given before saline did not affect θ(0) or MAX.
Conclusions: The decrease in θ(0) by morphine reflects its rewarding effects, which were attenuated by NK1R and opioid receptor blockade. These results demonstrate the importance of substance P signaling during limbic reward system activation by opioids.
Figures




Similar articles
-
Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine.Behav Brain Res. 2012 Sep 1;234(1):76-81. doi: 10.1016/j.bbr.2012.06.012. Epub 2012 Jun 21. Behav Brain Res. 2012. PMID: 22728726 Free PMC article.
-
Differential effects of aprepitant, a clinically used neurokinin-1 receptor antagonist on the expression of conditioned psychostimulant versus opioid reward.Psychopharmacology (Berl). 2017 Feb;234(4):695-705. doi: 10.1007/s00213-016-4504-6. Epub 2016 Dec 24. Psychopharmacology (Berl). 2017. PMID: 28013351 Free PMC article.
-
Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats.J Pharmacol Exp Ther. 2002 Feb;300(2):588-96. doi: 10.1124/jpet.300.2.588. J Pharmacol Exp Ther. 2002. PMID: 11805221
-
Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice.Psychopharmacology (Berl). 2010 Mar;209(1):103-11. doi: 10.1007/s00213-010-1775-1. Epub 2010 Jan 30. Psychopharmacology (Berl). 2010. PMID: 20112009
-
Targeting the Toll of Drug Abuse: The Translational Potential of Toll-Like Receptor 4.CNS Neurol Disord Drug Targets. 2015;14(6):692-9. doi: 10.2174/1871527314666150529132503. CNS Neurol Disord Drug Targets. 2015. PMID: 26022268 Free PMC article. Review.
Cited by
-
Gap Junctions Between Striatal D1 Neurons and Cholinergic Interneurons.Front Cell Neurosci. 2021 Jun 8;15:674399. doi: 10.3389/fncel.2021.674399. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34168539 Free PMC article.
-
A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior.Neuron. 2015 May 20;86(4):936-946. doi: 10.1016/j.neuron.2015.03.065. Epub 2015 Apr 30. Neuron. 2015. PMID: 25937170 Free PMC article.
-
The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking.Neuropsychopharmacology. 2014 Apr;39(5):1093-101. doi: 10.1038/npp.2013.309. Epub 2013 Oct 31. Neuropsychopharmacology. 2014. PMID: 24173499 Free PMC article.
-
Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine.Behav Brain Res. 2012 Sep 1;234(1):76-81. doi: 10.1016/j.bbr.2012.06.012. Epub 2012 Jun 21. Behav Brain Res. 2012. PMID: 22728726 Free PMC article.
-
Receptor Reserve Moderates Mesolimbic Responses to Opioids in a Humanized Mouse Model of the OPRM1 A118G Polymorphism.Neuropsychopharmacology. 2015 Oct;40(11):2614-22. doi: 10.1038/npp.2015.109. Epub 2015 Apr 16. Neuropsychopharmacology. 2015. PMID: 25881115 Free PMC article.
References
-
- Adams WJ, Lorens SA, Mitchell CL. Morphine enhances lateral hypothalamic self-stimulation in the rat. Proc Soc Exp Biol Med. 1972;140:770–1. - PubMed
-
- Bendani T, Cazala P. Differential effects of intracerebral microinjection of morphine on approach and escape responses induced by lateral hypothalamic stimulation in the mouse. Pharmacol Biochem Behav. 1988;30:397–401. - PubMed
-
- Boix F, Sandor P, Nogueira PJ, Huston JP, Schwarting RK. Relationship between dopamine release in nucleus accumbens and place preference induced by substance P injected into the nucleus basalis magnocellularis region. Neuroscience. 1995;64:1045–55. - PubMed
-
- Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement. NIDA Res Monogr. 1986;67:190–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

