Fetal programming and metabolic syndrome
- PMID: 21910625
- PMCID: PMC4132057
- DOI: 10.1146/annurev-physiol-020911-153245
Fetal programming and metabolic syndrome
Abstract
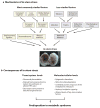
Metabolic syndrome is reaching epidemic proportions, particularly in developing countries. In this review, we explore the concept-based on the developmental-origin-of-health-and-disease hypothesis-that reprogramming during critical times of fetal life can lead to metabolic syndrome in adulthood. Specifically, we summarize the epidemiological evidence linking prenatal stress, manifested by low birth weight, to metabolic syndrome and its individual components. We also review animal studies that suggest potential mechanisms for the long-term effects of fetal reprogramming, including the cellular response to stress and both organ- and hormone-specific alterations induced by stress. Although metabolic syndrome in adulthood is undoubtedly caused by multiple factors, including modifiable behavior, fetal life may provide a critical window in which individuals are predisposed to metabolic syndrome later in life.
Figures

References
-
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. - PubMed
-
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. - PubMed
-
- Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–10. - PubMed
-
- Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

