The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I:C) and murine autoimmune cholangitis
- PMID: 21910728
- PMCID: PMC3193926
- DOI: 10.1111/j.1365-2249.2011.04453.x
The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I:C) and murine autoimmune cholangitis
Abstract
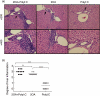
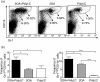
A void in understanding primary biliary cirrhosis (PBC) is the absence of appropriate animal models. Our laboratory has studied a murine model of autoimmune cholangitis induced following immunization with 2-octynoic acid (2OA), an antigen identified following extensive quantitative structural activity relationship (QSAR) analysis, using human autoantibodies and three-dimensional analysis of the mitochondrial autoantigen, the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2). Mice immunized with 2OA coupled to bovine serum albumin (BSA) develop anti-mitochondrial antibodies (AMAs) of the identical specificity as humans with PBC, and in addition develop inflammatory portal cell infiltrates in liver. However, the natural history of disease is less severe than in humans and does not include fibrosis. Data from human and autoimmune murine models suggest that environmental and/or infectious agents can exacerbate autoimmune reactions, and a model of PBC has been described in which polyinosinic-polycytidylic acid (poly I:C), a viral RNA mimetic and Toll-like receptor 3 (TLR-3) agonist induces low-titre AMAs and in mild portal infiltrates. We took advantage of our established model to determine whether immunization with 2OA-BSA coupled with poly I:C alters the disease process. Indeed, the addition of poly I:C produces a profound exacerbation of autoimmune cholangitis, including a significant increase in CD8(+) infiltrating T cells, as well as a marked increase of proinflammatory cytokines. In addition, mice have evidence of fibrosis. These findings lend support to the concept that besides breakdown of self-tolerance, there is a requirement of a second 'hit' during the breakdown process that leads to disease which more faithfully mimics human PBC.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures



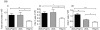
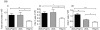
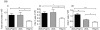

References
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–73. - PubMed
-
- Ueno Y, Ambrosini YM, Moritoki Y, Ridgway WM, Gershwin ME. Murine models of autoimmune cholangitis. Curr Opin Gastroenterol. 2010;26:274–9. - PubMed
-
- Amano K, Leung PS, Rieger R, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

