Characterization of nectin processing mediated by presenilin-dependent γ-secretase
- PMID: 21910732
- PMCID: PMC3217175
- DOI: 10.1111/j.1471-4159.2011.07479.x
Characterization of nectin processing mediated by presenilin-dependent γ-secretase
Abstract
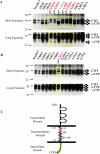
Nectins play an important role in forming various intercellular junctions including synapses. This role is regulated by several secretases present at intercellular junctions. We have investigated presenilin (PS)-dependent secretase-mediated processing of nectins in PS1 KO cells and primary hippocampal neurons. The loss of PS1/γ-secretase activity delayed the processing of nectin-1 and caused the accumulation of its full-length and C-terminal fragments. Over-expression of PS2 in PS1 KO cells compensated for the loss of PS1, suggesting that PS2 also has the ability to regulate nectin-1 processing. In mouse brain slices, a pronounced increase in levels of 30 and 24 kDa C-terminal fragments in response to chemical long-term potentiation was observed. The mouse brain synaptosomal fractionation study indicated that nectin-1 localized to post-synaptic and preferentially pre-synaptic membranes and that shedding occurs in both compartments. These data suggest that nectin-1 shedding and PS-dependent intramembrane cleavage occur at synapses, and is a regulated event during conditions of synaptic plasticity in the brain. Point mutation analysis identified several residues within the transmembrane domain that play a critical role in the positioning of cleavage sites by ectodomain sheddases. Nectin-3, which forms hetero-trans-dimers with nectin-1, also undergoes intramembrane cleavage mediated by PS1/γ-secretase, suggesting that PS1/γ-secreatse activity regulates synapse formation and remodeling by nectin processing.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures





References
-
- Berechid BE, Thinakaran G, Wong PC, Sisodia SS, Nye JS. Lack of requirement for presenilin1 in Notch1 signaling. Curr Biol. 1999;9:1493–1496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

