Secretagogues of lung surfactant increase annexin A7 localization with ABCA3 in alveolar type II cells
- PMID: 21911013
- PMCID: PMC3217159
- DOI: 10.1016/j.bbamcr.2011.07.022
Secretagogues of lung surfactant increase annexin A7 localization with ABCA3 in alveolar type II cells
Abstract
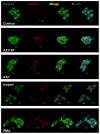
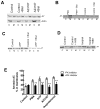
Membrane fusion between the lamellar bodies and plasma membrane is an obligatory event in the secretion of lung surfactant. Previous studies have postulated a role for annexin A7 (A7) in membrane fusion during exocytosis in some cells including alveolar type II cells. However, the intracellular trafficking of A7 during such fusion is not described. In this study, we investigated association of endogenous A7 with lamellar bodies in alveolar type II cells following treatment with several secretagogues of lung surfactant. Biochemical studies with specific antibodies showed increased membrane-association of cell A7 in type II cells stimulated with agents that increase secretion through different signaling mechanisms. Immuno-fluorescence studies showed increased co-localization of A7 with ABCA3, the lamellar body marker protein. Because these agents increase surfactant secretion through activation of PKC and PKA, we also investigated the effects of PKC and PKA inhibitors, bisindolylmaleimideI (BisI) and H89, respectively, on A7 partitioning. Western blot analysis showed that these inhibitors prevented secretagogue-mediated A7 increase in the membrane fractions. These inhibitors also blocked increased co-localization of A7 with ABCA3 in secretagogue-treated cells, as revealed by immuno-fluorescence studies. In vitro studies with recombinant A7 showed phosphorylation with PKC and PKA. The cell A7 was also phosphorylated in cells treated with surfactant secretagogues. Thus, our studies demonstrate that annexin A7 relocates to lamellar bodies in a phosphorylation-dependent manner. We suggest that activation of protein kinase promotes phosphorylation and membrane-association of A7 presumably to facilitate membrane fusion during lung surfactant secretion.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Annexin A7 and SNAP23 interactions in alveolar type II cells and in vitro: a role for Ca(2+) and PKC.Biochim Biophys Acta. 2012 Oct;1823(10):1796-806. doi: 10.1016/j.bbamcr.2012.06.010. Epub 2012 Jun 16. Biochim Biophys Acta. 2012. PMID: 22713544 Free PMC article.
-
Annexin A7 trafficking to alveolar type II cell surface: possible roles for protein insertion into membranes and lamellar body secretion.Biochim Biophys Acta. 2013 May;1833(5):1244-55. doi: 10.1016/j.bbamcr.2013.02.006. Epub 2013 Feb 19. Biochim Biophys Acta. 2013. PMID: 23434680 Free PMC article.
-
Calcium ionophore and phorbol ester increase membrane binding of annexin a7 in alveolar type II cells.Cell Calcium. 2003 Jan;33(1):11-7. doi: 10.1016/s0143-4160(02)00177-x. Cell Calcium. 2003. PMID: 12526883
-
Regulation of surfactant secretion in alveolar type II cells.Am J Physiol Lung Cell Mol Physiol. 2007 Aug;293(2):L259-71. doi: 10.1152/ajplung.00112.2007. Epub 2007 May 11. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17496061 Review.
-
The conception of fusion pores as rate-limiting structures for surfactant secretion.Comp Biochem Physiol A Mol Integr Physiol. 2001 May;129(1):227-31. doi: 10.1016/s1095-6433(01)00319-1. Comp Biochem Physiol A Mol Integr Physiol. 2001. PMID: 11369547 Review.
Cited by
-
Annexin A7 and SNAP23 interactions in alveolar type II cells and in vitro: a role for Ca(2+) and PKC.Biochim Biophys Acta. 2012 Oct;1823(10):1796-806. doi: 10.1016/j.bbamcr.2012.06.010. Epub 2012 Jun 16. Biochim Biophys Acta. 2012. PMID: 22713544 Free PMC article.
-
Surfactant phospholipid metabolism.Biochim Biophys Acta. 2013 Mar;1831(3):612-25. doi: 10.1016/j.bbalip.2012.09.010. Epub 2012 Sep 29. Biochim Biophys Acta. 2013. PMID: 23026158 Free PMC article. Review.
-
Spectral phasor analysis of LAURDAN fluorescence in live A549 lung cells to study the hydration and time evolution of intracellular lamellar body-like structures.Biochim Biophys Acta. 2016 Nov;1858(11):2625-2635. doi: 10.1016/j.bbamem.2016.07.017. Epub 2016 Jul 30. Biochim Biophys Acta. 2016. PMID: 27480804 Free PMC article.
-
Actin and Myosin in Non-Neuronal Exocytosis.Cells. 2020 Jun 11;9(6):1455. doi: 10.3390/cells9061455. Cells. 2020. PMID: 32545391 Free PMC article. Review.
-
Annexin A7 trafficking to alveolar type II cell surface: possible roles for protein insertion into membranes and lamellar body secretion.Biochim Biophys Acta. 2013 May;1833(5):1244-55. doi: 10.1016/j.bbamcr.2013.02.006. Epub 2013 Feb 19. Biochim Biophys Acta. 2013. PMID: 23434680 Free PMC article.
References
-
- Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:233–243. - PubMed
-
- Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L259–271. - PubMed
-
- Chander A, Fisher AB. Regulation of lung surfactant secretion. The American journal of physiology. 1990;258:L241–253. - PubMed
-
- Chander A, Wu RD. In vitro fusion of lung lamellar bodies and plasma membrane is augmented by lung synexin. Biochimica et biophysica acta. 1991;1086:157–166. - PubMed
-
- Chander A, Sen N, Spitzer AR. Synexin and GTP increase surfactant secretion in permeabilized alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L991–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

