Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo
- PMID: 21911374
- PMCID: PMC3179117
- DOI: 10.1073/pnas.1110444108
Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo
Abstract
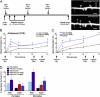
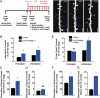
Glucocorticoids are a family of hormones that coordinate diverse physiological processes in responding to stress. Prolonged glucocorticoid exposure over weeks has been linked to dendritic atrophy and spine loss in fixed tissue studies of adult brains, but it is unclear how glucocorticoids may affect the dynamic processes of dendritic spine formation and elimination in vivo. Furthermore, relatively few studies have examined the effects of stress and glucocorticoids on spines during the postnatal and adolescent period, which is characterized by rapid synaptogenesis followed by protracted synaptic pruning. To determine whether and to what extent glucocorticoids regulate dendritic spine development and plasticity, we used transcranial two-photon microscopy to track the formation and elimination of dendritic spines in vivo after treatment with glucocorticoids in developing and adult mice. Corticosterone, the principal murine glucocorticoid, had potent dose-dependent effects on dendritic spine dynamics, increasing spine turnover within several hours in the developing barrel cortex. The adult barrel cortex exhibited diminished baseline spine turnover rates, but these rates were also enhanced by corticosterone. Similar changes occurred in multiple cortical areas, suggesting a generalized effect. However, reducing endogenous glucocorticoid activity by dexamethasone suppression or corticosteroid receptor antagonists caused a substantial reduction in spine turnover rates, and the former was reversed by corticosterone replacement. Notably, we found that chronic glucocorticoid excess led to an abnormal loss of stable spines that were established early in life. Together, these findings establish a critical role for glucocorticoids in the development and maintenance of dendritic spines in the living cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. - PubMed
-
- Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. - PubMed
-
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. - PubMed
-
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

