Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15
- PMID: 21911477
- PMCID: PMC3292392
- DOI: 10.1093/molehr/gar056
Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15
Abstract
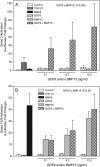
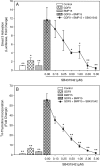
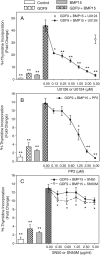
Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are two proteins selectively expressed in the oocyte which are essential for normal fertility. Both of these proteins are members of the transforming growth factor beta (TGF-β) superfamily and as such are produced as pre-proproteins, existing after proteolytic processing as a complex of the respective pro and mature regions. Previous work has shown that these two proteins interact both at the genetic and cellular signalling levels. In this study, our aim was to determine if the purified mature regions of GDF9 and BMP15 exhibit synergistic interactions on granulosa cells and to determine if such interactions are specific to these two proteins. We have used primary cultures of murine granulosa cells and [(3)H]-thymidine incorporation or transcriptional reporter assays as our readouts. We observed clear synergistic interactions between the mature regions of GDF9 and BMP15 when either DNA synthesis or SMAD3 signalling were examined. GDF9/BMP15 synergistic interactions were specific such that neither factor could be replaced by an analogous TGF-β superfamily member. The GDF9/BMP15 synergistic signalling response was inhibited by the SMAD2/3 phosphorylation inhibitor SB431542, as well as inhibition of the mitogen-activated protein kinase or rous sarcoma oncogene (SRC) signalling pathways, but not the nuclear factor kappa B pathway. In this study, we show that purified mature regions of GDF9 and BMP15 synergistically interact in a specific manner which is not dependent on the presence of a pro-region. This synergistic interaction is targeted at the SMAD3 pathway, and is dependent on ERK1/2 and SRC kinase signalling.
Figures


References
-
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 2006;119:408–415. - PubMed
-
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. - PubMed
-
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. - PubMed
-
- Edwards SJ, Reader KL, Lun S, Western A, Lawrence S, McNatty KP, Juengel JL. The cooperative effect of growth and differentiation factor-9 and bone morphogenetic protein (BMP)-15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology. 2008;149:1026–1030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

