Potassium-selective block of barium permeation through single KcsA channels
- PMID: 21911483
- PMCID: PMC3182450
- DOI: 10.1085/jgp.201110684
Potassium-selective block of barium permeation through single KcsA channels
Abstract
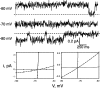
Ba(2+), a doubly charged analogue of K(+), specifically blocks K(+) channels by virtue of electrostatic stabilization in the permeation pathway. Ba(2+) block is used here as a tool to determine the equilibrium binding affinity for various monovalent cations at specific sites in the selectivity filter of a noninactivating mutant of KcsA. At high concentrations of external K(+), the block-time distribution is double exponential, marking at least two Ba(2+) sites in the selectivity filter, in accord with a Ba(2+)-containing crystal structure of KcsA. By analyzing block as a function of extracellular K(+), we determined the equilibrium dissociation constant of K(+) and of other monovalent cations at an extracellular site, presumably S1, to arrive at a selectivity sequence for binding at this site: Rb(+) (3 µM) > Cs(+) (23 µM) > K(+) (29 µM) > NH(4)(+) (440 µM) >> Na(+) and Li(+) (>1 M). This represents an unusually high selectivity for K(+) over Na(+), with |ΔΔG(0)| of at least 7 kcal mol(-1). These results fit well with other kinetic measurements of selectivity as well as with the many crystal structures of KcsA in various ionic conditions.
Figures








Similar articles
-
The conserved potassium channel filter can have distinct ion binding profiles: structural analysis of rubidium, cesium, and barium binding in NaK2K.J Gen Physiol. 2014 Aug;144(2):181-92. doi: 10.1085/jgp.201411191. Epub 2014 Jul 14. J Gen Physiol. 2014. PMID: 25024267 Free PMC article.
-
Selective exclusion and selective binding both contribute to ion selectivity in KcsA, a model potassium channel.J Biol Chem. 2017 Sep 15;292(37):15552-15560. doi: 10.1074/jbc.M117.795807. Epub 2017 Aug 4. J Biol Chem. 2017. PMID: 28778926 Free PMC article.
-
Sodium permeability and sensitivity induced by mutations in the selectivity filter of the KcsA channel towards Kir channels.Biochimie. 2010 Mar;92(3):232-44. doi: 10.1016/j.biochi.2009.11.007. Epub 2009 Dec 3. Biochimie. 2010. PMID: 19962419
-
Potassium channels: structures, models, simulations.Biochim Biophys Acta. 2002 Oct 11;1565(2):294-307. doi: 10.1016/s0005-2736(02)00576-x. Biochim Biophys Acta. 2002. PMID: 12409202 Review.
-
Ionic channels in epithelial cell membranes.Physiol Rev. 1985 Oct;65(4):833-903. doi: 10.1152/physrev.1985.65.4.833. Physiol Rev. 1985. PMID: 2414790 Review.
Cited by
-
Identification of a G-Protein-Independent Activator of GIRK Channels.Cell Rep. 2020 Jun 16;31(11):107770. doi: 10.1016/j.celrep.2020.107770. Cell Rep. 2020. PMID: 32553165 Free PMC article.
-
Inactivation in the potassium channel KcsA.J Struct Biol X. 2019 Jun 12;3:100009. doi: 10.1016/j.yjsbx.2019.100009. eCollection 2019 Jul-Sep. J Struct Biol X. 2019. PMID: 32647814 Free PMC article.
-
Designer and natural peptide toxin blockers of the KcsA potassium channel identified by phage display.Proc Natl Acad Sci U S A. 2015 Dec 15;112(50):E7013-21. doi: 10.1073/pnas.1514728112. Epub 2015 Dec 1. Proc Natl Acad Sci U S A. 2015. PMID: 26627718 Free PMC article.
-
Selectivity filter ion binding affinity determines inactivation in a potassium channel.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29968-29978. doi: 10.1073/pnas.2009624117. Epub 2020 Nov 5. Proc Natl Acad Sci U S A. 2020. PMID: 33154158 Free PMC article.
-
K⁺ conduction and Mg²⁺ blockade in a shaker Kv-channel single point mutant with an unusually high conductance.Biophys J. 2012 Sep 19;103(6):1198-207. doi: 10.1016/j.bpj.2012.08.015. Biophys J. 2012. PMID: 22995492 Free PMC article.
References
-
- Bernardo J.M., Smith A.F.M. 1994. Bayesian Theory. Wiley, Chichester, England: 586 pp
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

