The role of in vivo Ca²⁺ signals acting on Ca²⁺-calmodulin-dependent proteins for skeletal muscle plasticity
- PMID: 21911615
- PMCID: PMC3225663
- DOI: 10.1113/jphysiol.2011.212860
The role of in vivo Ca²⁺ signals acting on Ca²⁺-calmodulin-dependent proteins for skeletal muscle plasticity
Abstract
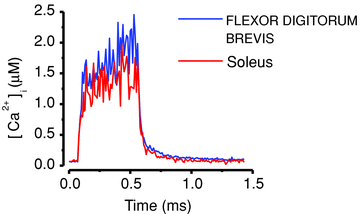
Skeletal muscle fibres are highly heterogeneous regarding size, metabolism and contractile function. They also show a large capacity for adaptations in response to alterations in the activation pattern. A major part of this activity-dependent plasticity relies on transcriptional alterations controlled by intracellular Ca(2+) signals. In this review we discuss how intracellular Ca(2+) fluctuations induced by activation patterns likely to occur in vivo control muscle properties via effects on Ca(2+)-calmodulin-dependent proteins. We focus on two such Ca(2+) decoders: calcineurin and Ca(2+)-calmodulin-dependent protein kinase II. Inherent Ca(2+) transients during contractions differ rather little between slow- and fast-twitch muscle fibres and this difference is unlikely to have any significant impact on the activity of Ca(2+) decoders. The major exception to this is fatigue-induced changes in Ca(2+) transients that occur in fast-twitch fibres exposed to high-intensity activation typical of slow-twitch motor units. In conclusion, the cascade from neural stimulation pattern to Ca(2+)-dependent transcription is likely to be central in maintaining the fibre phenotypes in both fast- and slow-twitch fibres. Moreover, changes in Ca(2+) signalling (e.g. induced by endurance training) can result in altered muscle properties (e.g. increased mitochondrial biogenesis) and this plasticity involves other signalling pathways.
Figures



References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. - PubMed
-
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. - PubMed
-
- Anderson ME, Erickson JR, Joiner MLA, Guan X, Kutschke W, Yang JY, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. - PMC - PubMed
-
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum Mol Genet. 2009;18:278–288. - PubMed
-
- Aydin J, Korhonen T, Tavi P, Allen DG, Westerblad H, Bruton JD. Activation of Ca2+-dependent protein kinase II during repeated contractions in single muscle fibres from mouse is dependent on the frequency of sarcoplasmic reticulum Ca2+ release. Acta Physiol (Oxf) 2007;191:131–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

