Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial
- PMID: 21911655
- PMCID: PMC3260944
- DOI: 10.1001/archneurol.2011.233
Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial
Abstract
Objective: To examine the effects of intranasal insulin administration on cognition, function, cerebral glucose metabolism, and cerebrospinal fluid biomarkers in adults with amnestic mild cognitive impairment or Alzheimer disease (AD).
Design: Randomized, double-blind, placebo-controlled trial.
Setting: Clinical research unit of a Veterans Affairs medical center.
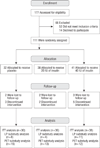
Participants: The intent-to-treat sample consisted of 104 adults with amnestic mild cognitive impairment (n = 64) or mild to moderate AD (n = 40). Intervention Participants received placebo (n = 30), 20 IU of insulin (n = 36), or 40 IU of insulin (n = 38) for 4 months, administered with a nasal drug delivery device (Kurve Technology, Bothell, Washington).
Main outcome measures: Primary measures consisted of delayed story recall score and the Dementia Severity Rating Scale score, and secondary measures included the Alzheimer Disease's Assessment Scale-cognitive subscale (ADAS-cog) score and the Alzheimer's Disease Cooperative Study-activities of daily living (ADCS-ADL) scale. A subset of participants underwent lumbar puncture (n = 23) and positron emission tomography with fludeoxyglucose F 18 (n = 40) before and after treatment.
Results: Outcome measures were analyzed using repeated-measures analysis of covariance. Treatment with 20 IU of insulin improved delayed memory (P < .05), and both doses of insulin (20 and 40 IU) preserved caregiver-rated functional ability (P < .01). Both insulin doses also preserved general cognition as assessed by the ADAS-cog score for younger participants and functional abilities as assessed by the ADCS-ADL scale for adults with AD (P < .05). Cerebrospinal fluid biomarkers did not change for insulin-treated participants as a group, but, in exploratory analyses, changes in memory and function were associated with changes in the Aβ42 level and in the tau protein-to-Aβ42 ratio in cerebrospinal fluid. Placebo-assigned participants showed decreased fludeoxyglucose F 18 uptake in the parietotemporal, frontal, precuneus, and cuneus regions and insulin-minimized progression. No treatment-related severe adverse events occurred.
Conclusions: These results support longer trials of intranasal insulin therapy for patients with amnestic mild cognitive impairment and patients with AD. Trial Registration clinicaltrials.gov Identifier: NCT00438568.
Figures


Comment in
-
Insulin to treat Alzheimer's disease: just follow your nose?Expert Rev Clin Pharmacol. 2012 Jan;5(1):17-20. doi: 10.1586/ecp.11.70. Expert Rev Clin Pharmacol. 2012. PMID: 22142155
-
Insulin and Alzheimer disease.Arch Neurol. 2012 May;69(5):670-1; author reply 671. doi: 10.1001/archneurol.2012.42. Arch Neurol. 2012. PMID: 22782516 No abstract available.
References
-
- Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Bio-phys Acta. 2009;1792(5):482–496. - PubMed
-
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50(1):164–168. - PubMed
-
- Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

