Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy
- PMID: 21911743
- PMCID: PMC3198056
- DOI: 10.2337/db11-0358
Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy
Abstract
Objective: To assess the potential role of FoxP3-expressing regulatory T cells (Tregs) in reversing obesity-linked insulin resistance and diabetic nephropathy in rodent models and humans.
Research design and methods: To characterize the role of Tregs in insulin resistance, human visceral adipose tissue was first evaluated for Treg infiltration and second, the db/db mouse model was evaluated.
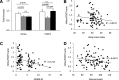
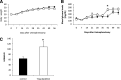
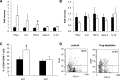
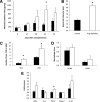
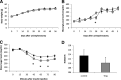
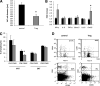
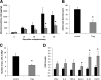
Results: Obese patients with insulin resistance displayed significantly decreased natural Tregs but an increase in adaptive Tregs in their visceral adipose tissue as compared with lean control subjects. To further evaluate the pathogenic role of Tregs in insulin resistance, the db/db mouse model was used. Treg depletion using an anti-CD25 monoclonal antibody enhanced insulin resistance as shown by increased fasting blood glucose levels as well as an impaired insulin sensitivity. Moreover, Treg-depleted db/db mice developed increased signs of diabetic nephropathy, such as albuminuria and glomerular hyperfiltration. This was paralleled by a proinflammatory milieu in both murine visceral adipose tissue and the kidney. Conversely, adoptive transfer of CD4(+)FoxP3(+) Tregs significantly improved insulin sensitivity and diabetic nephropathy. Accordingly, there was increased mRNA expression of FoxP3 as well as less abundant proinflammatory CD8(+)CD69(+) T cells in visceral adipose tissue and kidneys of Treg-treated animals.
Conclusions: Data suggest a potential therapeutic value of Tregs to improve insulin resistance and end organ damage in type 2 diabetes by limiting the proinflammatory milieu.
Figures
References
-
- Wolf D, Hochegger K, Wolf AM, et al. . CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol 2005;16:1360–1370 - PubMed
-
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003;170:3939–3943 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

