Absence of p53 gene expression in selenium molecular prevention of chemically induced hepatocarcinogenesis in rats
- PMID: 21912060
- PMCID: PMC3178921
- DOI: 10.4103/1319-3767.84489
Absence of p53 gene expression in selenium molecular prevention of chemically induced hepatocarcinogenesis in rats
Abstract
Background/aim: p53 pathway is thought by many researchers to be critically involved in selenium's chemoprevention or in hepatocarcinogenesis. The aim of this study was to investigate the gene expression of p53, p21 and B-cell lymphoma-2 (bcl-2) using preventive and therapeutic approaches of selenium in chemically induced hepatocarcinogenesis in rats.
Materials and methods: Rats were divided randomly into six groups: Negative control, positive control (diethyl nitrosamine +2-acetylaminofluorene), preventive group, preventive control (respective control for preventive group), therapeutic group and therapeutic control (respective control for therapeutic group). p53, p21 and bcl-2 genes on liver tissues were measured using real-time polymerase chain reaction.
Results: The expression of p53 was only significant in the therapeutic control. The expression of bcl-2 was insignificant in all the groups. p21 expression was significant in all the groups except the preventive group.
Conclusions: The selenium molecular mechanism for liver cancer prevention is not through the p53 pathway. Also, the absence of p53 is not necessary for chemically induced liver cancer in rats.
Conflict of interest statement
Figures




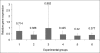

References
-
- Vousden K, Lane D. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. - PubMed
-
- Wei Y, Cao X, Ou Y, Lu J, Xing C, Zheng R. SeO2 induces apoptosis with down-regulation of Bcl-2 and up-regulation of p53 expression in both immortal human hepatic cell line and hepatoma cell line. Muta Res. 2001;490:113–21. - PubMed
-
- Kajta K. Apoptosis in the central nervous system: Mechanisms and protective strategies. Pol J Pharmacol. 2004;56:689–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
