Separation of recombination and SOS response in Escherichia coli RecA suggests LexA interaction sites
- PMID: 21912525
- PMCID: PMC3164682
- DOI: 10.1371/journal.pgen.1002244
Separation of recombination and SOS response in Escherichia coli RecA suggests LexA interaction sites
Abstract
RecA plays a key role in homologous recombination, the induction of the DNA damage response through LexA cleavage and the activity of error-prone polymerase in Escherichia coli. RecA interacts with multiple partners to achieve this pleiotropic role, but the structural location and sequence determinants involved in these multiple interactions remain mostly unknown. Here, in a first application to prokaryotes, Evolutionary Trace (ET) analysis identifies clusters of evolutionarily important surface amino acids involved in RecA functions. Some of these clusters match the known ATP binding, DNA binding, and RecA-RecA homo-dimerization sites, but others are novel. Mutation analysis at these sites disrupted either recombination or LexA cleavage. This highlights distinct functional sites specific for recombination and DNA damage response induction. Finally, our analysis reveals a composite site for LexA binding and cleavage, which is formed only on the active RecA filament. These new sites can provide new drug targets to modulate one or more RecA functions, with the potential to address the problem of evolution of antibiotic resistance at its root.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

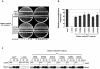



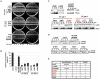

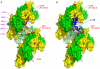
References
-
- Radding CM. Recombination activities of E. coli recA protein. Cell. 1981;25:3–4. - PubMed
-
- Lusetti SL, Cox MM. The bacterial RecA protein and the recombinational DNA repair of stalled replication forks. Annu Rev Biochem. 2002;71:71–100. - PubMed
-
- Cox MM. Regulation of bacterial RecA protein function. Crit Rev Biochem Mol Biol. 2007;42:41–63. - PubMed
-
- Cox MM. Motoring along with the bacterial RecA protein. Nat Rev Mol Cell Biol. 2007;8:127–138. - PubMed
-
- Tamas I, Klasson L, Canback B, Naslund AK, Eriksson AS, et al. 50 million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–2379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

