Tetraarsenic Hexoxide Induces Beclin-1-Induced Autophagic Cell Death as well as Caspase-Dependent Apoptosis in U937 Human Leukemic Cells
- PMID: 21912568
- PMCID: PMC3170805
- DOI: 10.1155/2012/201414
Tetraarsenic Hexoxide Induces Beclin-1-Induced Autophagic Cell Death as well as Caspase-Dependent Apoptosis in U937 Human Leukemic Cells
Abstract
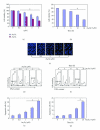
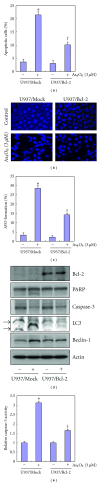
Tetraarsenic hexaoxide (As(4)O(6)) has been used in Korean folk remedy for the treatment of cancer since the late 1980s, and arsenic trioxide (As(2)O(3)) is currently used as a chemotherapeutic agent. However, evidence suggests that As(4)O(6)-induced cell death pathway was different from that of As(2)O(3). Besides, the anticancer effects and mechanisms of As(4)O(6) are not fully understood. Therefore, we investigated the anticancer activities of As(4)O(6) on apoptosis and autophagy in U937 human leukemic cells. The growth of U937 cells was inhibited by As(4)O(6) treatment in a dose- and a time-dependent manner, and IC(50) for As(4)O(6) was less than 2 μM. As(4)O(6) induced caspase-dependent apoptosis and Beclin-1-induced autophagy, both of which were significantly attenuated by Bcl-2 augmentation and N-acetylcysteine (NAC) treatment. This study suggests that As(4)O(6) should induce Beclin-1-induced autophagic cell death as well as caspase-dependent apoptosis and that it might be a promising agent for the treatment of leukemia.
Figures




References
-
- Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. - PubMed
-
- Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. - PubMed
-
- Munshi NC, Tricot G, Desikan R, et al. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia. 2002;16(9):1835–1837. - PubMed
-
- Lin YC, Li DR, Lin W. Relationship between radiotherapy enhancing effect of arsenic trioxide and the proliferation and apoptosis of related protein in nasopharyngeal carcinoma patients. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27(8):704–707. - PubMed
-
- Walker PR, Sikorska M. New aspects of the mechanism of DNA fragmentation in apoptosis. Biochemistry and Cell Biology. 1997;75(4):287–299. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

