Reduced neutrophil apoptosis in diabetic mice during staphylococcal infection leads to prolonged Tnfα production and reduced neutrophil clearance
- PMID: 21912601
- PMCID: PMC3166063
- DOI: 10.1371/journal.pone.0023633
Reduced neutrophil apoptosis in diabetic mice during staphylococcal infection leads to prolonged Tnfα production and reduced neutrophil clearance
Abstract
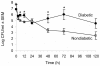
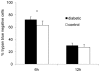
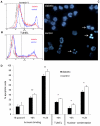
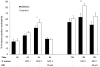
Diabetes is a frequent underlying medical condition among individuals with Staphylococcus aureus infections, and diabetic patients often suffer from chronic inflammation and prolonged infections. Neutrophils are the most abundant inflammatory cells during the early stages of bacterial diseases, and previous studies have reported deficiencies in neutrophil function in diabetic hosts. We challenged age-matched hyperglycemic and normoglycemic NOD mice intraperitoneally with S. aureus and evaluated the fate of neutrophils recruited to the peritoneal cavity. Neutrophils were more abundant in the peritoneal fluids of infected diabetic mice by 48 h after bacterial inoculation, and they showed prolonged viability ex vivo compared to neutrophils from infected nondiabetic mice. These differences correlated with reduced apoptosis of neutrophils from diabetic mice and were dependent upon the presence of S. aureus and a functional neutrophil respiratory burst. Decreased apoptosis correlated with impaired clearance of neutrophils by macrophages both in vitro and in vivo and prolonged production of proinflammatory tumor necrosis factor alpha by neutrophils from diabetic mice. Our results suggest that defects in neutrophil apoptosis may contribute to the chronic inflammation and the inability to clear staphylococcal infections observed in diabetic patients.
Conflict of interest statement
Figures




References
-
- Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. - PubMed
-
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
-
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. - PubMed
-
- Bader MS. Diabetic foot infection. Am Fam Physician. 2008;78:71–79. - PubMed
-
- Tuazon CU, Perez A, Kishaba T, Sheagren JN. Staphylococcus aureus among insulin-injecting diabetic patients. An increased carrier rate. JAMA. 1975;231:1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

