Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple myeloma cells and increases their susceptibility to Fas-mediated apoptosis
- PMID: 21912642
- PMCID: PMC3164673
- DOI: 10.1371/journal.pone.0023741
Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple myeloma cells and increases their susceptibility to Fas-mediated apoptosis
Abstract
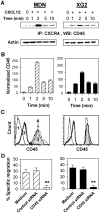
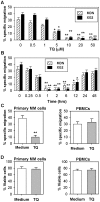
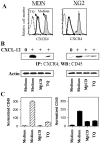
In multiple myeloma (MM), malignant plasma cells reside in the bone marrow, where they accumulate in close contact with stromal cells. The mechanisms responsible for the chemotaxis of malignant plasma cells are still poorly understood. Thus, we investigated the mechanisms involved in the chemotaxis of MDN and XG2 MM cell lines. Both cell lines strongly expressed CCR9, CXCR3 and CXCR4 chemokine receptors but only migrated toward CXCL12. Activation of CXCR4 by CXCL12 resulted in the association of CXCR4 with CD45 and activation of PLCβ3, AKT, RhoA, IκBα and ERK1/2. Using siRNA-silencing techniques, we showed CD45/CXCR4 association is essential for CXCL12-induced migration of MM cells. Thymoquinone (TQ), the major active component of the medicinal herb Nigella sativa Linn, has been described as a chemopreventive and chemotherapeutic compound. TQ treatment strongly inhibited CXCL12-mediated chemotaxis in MM cell lines as well as primary cells isolated from MM patients, but not normal PBMCs. Moreover, TQ significantly down-regulated CXCR4 expression and CXCL12-mediated CXCR4/CD45 association in MM cells. Finally, TQ also induced the relocalization of cytoplasmic Fas/CD95 to the membrane of MM cells and increased CD95-mediated apoptosis by 80%. In conclusion, we demonstrate the potent anti-myeloma activity of TQ, providing a rationale for further clinical evaluation.
Conflict of interest statement
Figures



References
-
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: emphasis on risk factors for progression. Br J Haematol. 2007;139:730–743. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Ishii Y, Hsiao HH, Sashida G, Ito Y, Miyazawa K, et al. Derivative (1;7)(q10;p10) in multiple myeloma. A sign of therapy-related hidden myelodysplastic syndrome. Cancer Genet Cytogenet. 2006;167:131–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

