Lysine392, a K63-linked ubiquitination site in NEMO, mediates inflammatory osteoclastogenesis and osteolysis
- PMID: 21913221
- PMCID: PMC3272311
- DOI: 10.1002/jor.21555
Lysine392, a K63-linked ubiquitination site in NEMO, mediates inflammatory osteoclastogenesis and osteolysis
Abstract
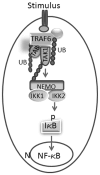
PMMA particles released from bone implants are considered major contributor to osteolysis and subsequent implant failure. Although the ensuing inflammatory response has been described, the mechanisms underlying PMMA particulate-induced osteolysis remain enigmatic. In previous studies, we have established that activation of Nuclear factor kappa-B (NF-κB) and MAP kinase pathways plays a central role in the pathogenesis of inflammatory osteolysis. Specifically, we have shown that impeding IKK complex assembly, and thus subsequent NF-κB activation, dampens particle-induced osteolysis. The IKK complex consists of IKKα, IKKβ, and IKKγ, also known as NEMO. NEMO has no catalytic activity and serves as a scaffold protein facilitating assembly and distal activation of NF-κB signaling. In fact, blocking binding of NEMO with IKKα/β abolishes NF-κB activity. In the current study, we identify Lysine 392 residue in NEMO as crucial mediator of PMMA particle-induced inflammatory osteoclastogenesis and osteolysis. Using mice in which NEMO-K392R mutation has been introduced, we provide evidence that PMMA-induced osteoclasts and osteolytic responses are impaired. Furthermore, we show that this impairment is likely due to poor activation of NF-κB and Erk, but not other MAP kinases. Our findings suggest that NEMO Lysine392, a well-established K63-linked polyubiquitination site, is an important mediator of PMMA-induced osteolysis. Therefore, this NEMO motif should be considered as a target to combat PMMA particle-induced osteolysis.
Copyright © 2011 Orthopaedic Research Society.
Figures





References
-
- Schwarz EM, O'Keefe RJ, Looney RJ. Bone implant interface, osteolysis and potential therapies. Journal of Musculoskeletal Neuronal Interactions. 2004;4:390–392. - PubMed
-
- Abu-Amer Y. Mechanisms of inflammatory mediators in bone loss diseases. In: Rosier RN, Evans CH, editors. Molecular biology in orthopedics. AAOS; 2003. pp. 229–239.
-
- Neale SD, Athanasou NA. Cytokine receptor profile of arthroplasty macrophages, foreign body giant cells and mature osteoclasts. Acta orthopaedica Scandinavica. 1999;70:452–458. - PubMed
-
- Ren W, Wu B, Peng X, Hua J, Hao HN, Wooley PH. Implant wear induces inflammation, but not osteoclastic bone resorption, in RANK(−/−) mice. J Orthop Res. 2006;24:1575–1586. - PubMed
-
- Sabokbar A, Itonaga I, Sun SG, Kudo O, Athanasou NA. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23:511–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

