Mass spectrometry based approach to study the kinetics of O6-alkylguanine DNA alkyltransferase-mediated repair of O6-pyridyloxobutyl-2'-deoxyguanosine adducts in DNA
- PMID: 21913712
- PMCID: PMC3221886
- DOI: 10.1021/tx2002993
Mass spectrometry based approach to study the kinetics of O6-alkylguanine DNA alkyltransferase-mediated repair of O6-pyridyloxobutyl-2'-deoxyguanosine adducts in DNA
Abstract
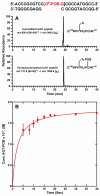
O(6)-POB-dG (O(6)-[4-oxo-4-(3-pyridyl)but-1-yl]deoxyguanosine) are promutagenic nucleobase adducts that arise from DNA alkylation by metabolically activated tobacco-specific nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosonicotine (NNN). If not repaired, O(6)-POB-dG adducts cause mispairing during DNA replication, leading to G → A and G → T mutations. A specialized DNA repair protein, O(6)-alkylguanine-DNA-alkyltransferase (AGT), transfers the POB group from O(6)-POB-dG in DNA to a cysteine residue within the protein (Cys145), thus restoring normal guanine and preventing mutagenesis. The rates of AGT-mediated repair of O(6)-POB-dG may be affected by local DNA sequence context, potentially leading to adduct accumulation and increased mutagenesis at specific sites within the genome. In the present work, isotope dilution high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI(+)-MS/MS)-based methodology was developed to investigate the influence of DNA sequence on the kinetics of AGT-mediated repair of O(6)-POB-dG adducts. In our approach, synthetic DNA duplexes containing O(6)-POB-dG at a specified site are incubated with recombinant human AGT protein for defined periods of time. Following spiking with D(4)-O(6)-POB-dG internal standard and mild acid hydrolysis to release O(6)-POB-guanine (O(6)-POB-G) and D(4)-O(6)-POB-guanine (D(4)-O(6)-POB-G), samples are purified by solid phase extraction (SPE), and O(6)-POB-G adducts remaining in DNA are quantified by capillary HPLC-ESI(+)-MS/MS. The new method was validated by analyzing mixtures containing known amounts of O(6)-POB-G-containig DNA and the corresponding unmodified DNA duplexes and by examining the kinetics of alkyl transfer in the presence of increasing amounts of AGT protein. The disappearance of O(6)-POB-dG from DNA was accompanied by pyridyloxobutylation of AGT Cys-145 as determined by HPLC-ESI(+)-MS/MS of tryptic peptides. The applicability of the new approach was shown by determining the second order kinetics of AGT-mediated repair of O(6)-POB-dG adducts placed within a DNA duplex representing modified rat H-ras sequence (5'-AATAGTATCT[O(6)-POB-G]GAGCC-3') opposite either C or T. Faster rates of alkyl transfer were observed when O(6)-POB-dG was paired with T rather than with C (k = 1.74 × 10(6) M(-1) s(-1) vs 1.17 × 10(6) M(-1) s(-1)).
Figures






References
-
- Hecht SS, Chen CB, Ohmori T, Hoffmann D. Comparative carcinogenicity in F344 rats of the tobacco-specific nitrosamines, N’-nitrosonornicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1980;40:298–302. - PubMed
-
- Hecht SS, Morse MA, Amin S, Stoner GD, Jordan KG, Choi CI, Chung FL. Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis. 1989;10:1901–1904. - PubMed
-
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. - PubMed
-
- Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. 1999;424:127–142. - PubMed
-
- Pauly GT, Peterson LA, Moschel RC. Mutagenesis by O6-[4-oxo-4-(3-pyridyl)butyl]guanine in Esherichia coli and human cells. Chem. Res. Toxicol. 2002;15:165–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

