Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase
- PMID: 21913897
- PMCID: PMC3372732
- DOI: 10.1111/j.1476-5381.2011.01666.x
Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase
Abstract
Background and purposes: Thienyl-isoquinolone (TIQ-A) is a relatively potent PARP inhibitor able to reduce post-ischaemic neuronal death in vitro. Here we have studied, in different stroke models in vivo, the neuroprotective properties of DAMTIQ and HYDAMTIQ, two TIQ-A derivatives able to reach the brain and to inhibit PARP-1 and PARP-2.
Experimental approach: Studies were carried out in (i) transient (2 h) middle cerebral artery occlusion (tMCAO), (ii) permanent MCAO (pMCAO) and (iii) electrocoagulation of the distal portion of MCA in conjunction with transient (90 min) bilateral carotid occlusion (focal cortical ischaemia).
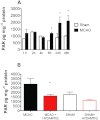
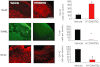
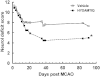
Key results: In male rats with tMCAO, HYDAMTIQ (0.1-10 mg·kg(-1)) injected i.p. three times, starting 4 h after MCAO, reduced infarct volumes by up to 70%, reduced the loss of body weight by up to 60% and attenuated the neurological impairment by up to 40%. In age-matched female rats, HYDAMTIQ also reduced brain damage. Protection, however, was less pronounced than in the male rats. In animals with pMCAO, HYDAMTIQ administered 30 min after MCAO reduced infarct volumes by approximately 40%. In animals with focal cortical ischaemia, HYDAMTIQ treatment decreased post-ischaemic accumulation of PAR (the product of PARP activity) and the presence of OX42-positive inflammatory cells in the ischaemic cortex. It also reduced sensorimotor deficits for up to 90 days after MCAO.
Conclusion and implications: Our results show that HYDAMTIQ is a potent PARP inhibitor that conferred robust neuroprotection and long-lasting improvement of post-stroke neurological deficits.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures







References
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
-
- Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. - PubMed
-
- Caiafa P, Guastafierro T, Zampieri M. Epigenetics: poly(ADP-ribosyl)ation of PARP-1 regulates genomic methylation patterns. FASEB J. 2009;23:672–678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

