Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: open-label extension of the ATTEST Study
- PMID: 21914628
- PMCID: PMC3184243
- DOI: 10.1136/annrheumdis-2011-200316
Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: open-label extension of the ATTEST Study
Abstract
Objective: To assess the efficacy and safety of abatacept in biological-naive patients with rheumatoid arthritis and an inadequate response to methotrexate treated in the long-term extension (LTE) of the ATTEST trial.
Methods: Patients randomly assigned to abatacept, placebo or infliximab completing the 1-year double-blind period were eligible to receive abatacept ∼10 mg/kg in the open-label LTE. Efficacy to year 2 is presented for patients randomly assigned to abatacept or infliximab who switched to open-label abatacept. Safety data are presented for all patients entering LTE regardless of double-blind treatment.
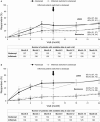
Results: Of 431 patients randomly assigned, 79.8% remained on abatacept at year 2. At years 1 and 2, 19.7% and 26.1% of abatacept and 13.3% and 28.6% of infliximab-to-abatacept patients achieved disease activity score 28-defined remission (<2.6). Safety with abatacept during the cumulative study period was consistent with the double-blind experience, with no increase in adverse event incidence following the switch to abatacept.
Conclusion: In methotrexate-inadequate responders, abatacept efficacy was maintained over 2 years. For infliximab-to-abatacept patients, efficacy improvements were seen in year 2 after patients switched to abatacept. Switching directly from infliximab to abatacept was well tolerated. These data demonstrate that abatacept provides sustained responses and consistent safety, suggesting that switching from infliximab to abatacept is a viable treatment option.
Conflict of interest statement
Figures
References
-
- Genovese MC, Schiff M, Luggen M, et al. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis 2008;67:547–54 - PubMed
-
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793–806 - PubMed
-
- Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516–23 - PMC - PubMed
-
- Finckh A, Ciurea A, Brulhart L, et al. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum 2007;56:1417–23 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

