Implication of low level inflammation in the insulin resistance of adipose tissue at late pregnancy
- PMID: 21914778
- PMCID: PMC3198999
- DOI: 10.1210/en.2011-0068
Implication of low level inflammation in the insulin resistance of adipose tissue at late pregnancy
Abstract
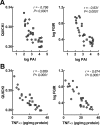
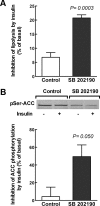
Insulin resistance is a characteristic of late pregnancy, and adipose tissue is one of the tissues that most actively contributes to the reduced maternal insulin sensitivity. There is evidence that pregnancy is a condition of moderate inflammation, although the physiological role of this low-grade inflammation remains unclear. The present study was designed to validate whether low-grade inflammation plays a role in the development of insulin resistance in adipose tissue during late pregnancy. To this end, we analyzed proinflammatory adipokines and kinases in lumbar adipose tissue of nonpregnant and late pregnant rats at d 18 and 20 of gestation. We found that circulating and tissue levels of adipokines, such as IL-1β, plasminogen activator inhibitor-1, and TNF-α, were increased at late pregnancy, which correlated with insulin resistance. The observed increase in adipokines coincided with an enhanced activation of p38 MAPK in adipose tissue. Treatment of pregnant rats with the p38 MAPK inhibitor SB 202190 increased insulin-stimulated tyrosine phosphorylation of the insulin receptor (IR) and IR substrate-1 in adipose tissue, which was paralleled by a reduction of IR substrate-1 serine phosphorylation and an enhancement of the metabolic actions of insulin. These results indicate that activation of p38 MAPK in adipose tissue contributes to adipose tissue insulin resistance at late pregnancy. Furthermore, the results of the present study support the hypothesis that physiological low-grade inflammation in the maternal organism is relevant to the development of pregnancy-associated insulin resistance.
Figures




References
-
- Flier JS. 1995. The adipocyte: storage depot or node on the energy information superhighway? Cell 80:15–18 - PubMed
-
- Sjöholm A, Nyström T. 2006. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev 22:4–10 - PubMed
-
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. 2005. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426–430 - PubMed

