PGC1α promotes tumor growth by inducing gene expression programs supporting lipogenesis
- PMID: 21914785
- PMCID: PMC3282487
- DOI: 10.1158/0008-5472.CAN-11-1011
PGC1α promotes tumor growth by inducing gene expression programs supporting lipogenesis
Abstract
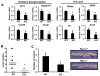
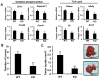
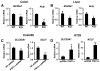
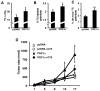
Despite the role of aerobic glycolysis in cancer, recent studies highlight the importance of the mitochondria and biosynthetic pathways as well. PPARγ coactivator 1α (PGC1α) is a key transcriptional regulator of several metabolic pathways including oxidative metabolism and lipogenesis. Initial studies suggested that PGC1α expression is reduced in tumors compared with adjacent normal tissue. Paradoxically, other studies show that PGC1α is associated with cancer cell proliferation. Therefore, the role of PGC1α in cancer and especially carcinogenesis is unclear. Using Pgc1α(-/-) and Pgc1α(+/+) mice, we show that loss of PGC1α protects mice from azoxymethane-induced colon carcinogenesis. Similarly, diethylnitrosamine-induced liver carcinogenesis is reduced in Pgc1α(-/-) mice as compared with Pgc1α(+/+) mice. Xenograft studies using gain and loss of PGC1α expression showed that PGC1α also promotes tumor growth. Interestingly, while PGC1α induced oxidative phosphorylation and tricarboxylic acid cycle gene expression, we also observed an increase in the expression of two genes required for de novo fatty acid synthesis, ACC and FASN. In addition, SLC25A1 and ACLY, which are required for the conversion of glucose into acetyl-CoA for fatty acid synthesis, were also increased by PGC1α, thus linking the oxidative and lipogenic functions of PGC1α. Indeed, using stable (13)C isotope tracer analysis, we show that PGC1α increased de novo lipogenesis. Importantly, inhibition of fatty acid synthesis blunted these progrowth effects of PGC1α. In conclusion, these studies show for the first time that loss of PGC1α protects against carcinogenesis and that PGC1α coordinately regulates mitochondrial and fatty acid metabolism to promote tumor growth.
©2011 AACR.
Figures



References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. - PubMed
-
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews. 2007;7:763–77. - PubMed
-
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

