Repressor transcription factor 7-like 1 promotes adipogenic competency in precursor cells
- PMID: 21914845
- PMCID: PMC3182685
- DOI: 10.1073/pnas.1109409108
Repressor transcription factor 7-like 1 promotes adipogenic competency in precursor cells
Abstract
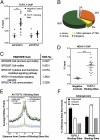
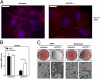
The identification of factors that define adipocyte precursor potential has important implications for obesity. Preadipocytes are fibroblastoid cells committed to becoming round lipid-laden adipocytes. In vitro, this differentiation process is facilitated by confluency, followed by adipogenic stimuli. During adipogenesis, a large number of cytostructural genes are repressed before adipocyte gene induction. Here we report that the transcriptional repressor transcription factor 7-like 1 (TCF7L1) binds and directly regulates the expression of cell structure genes. Depletion of TCF7L1 inhibits differentiation, because TCF7L1 indirectly induces the adipogenic transcription factor peroxisome proliferator-activated receptor γ in a manner that can be replaced by inhibition of myosin II activity. TCF7L1 is induced by cell contact in adipogenic cell lines, and ectopic expression of TCF7L1 alleviates the confluency requirement for adipocytic differentiation of precursor cells. In contrast, TCF7L1 is not induced during confluency of non-adipogenic fibroblasts, and, remarkably, forced expression of TCF7L1 is sufficient to commit non-adipogenic fibroblasts to an adipogenic fate. These results establish TCF7L1 as a transcriptional hub coordinating cell-cell contact with the transcriptional repression required for adipogenic competency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

