Novel synthesis of pseudopeptides bearing a difluoromethyl group by Ugi reaction and desulfanylation
- PMID: 21915210
- PMCID: PMC3170163
- DOI: 10.3762/bjoc.7.123
Novel synthesis of pseudopeptides bearing a difluoromethyl group by Ugi reaction and desulfanylation
Abstract
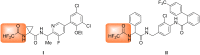
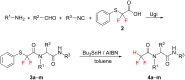
Thirteen difluoromethyl-containing pseudopeptides were synthesized by Ugi reaction using the novel building block 2,2-difluoro-2-(phenylthio)acetic acid (2) as one component, followed by removal of the phenylsulfanyl protecting group in the presence of tributyltin hydride and azobisisobutyronitrile.
Keywords: Ugi reaction; difluoromethyl functionality; gem-difluoromethylene-containing acid; pseudopeptides; reductive cleavage.
Figures

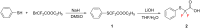
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
