Differential temporal and spatial progerin expression during closure of the ductus arteriosus in neonates
- PMID: 21915271
- PMCID: PMC3167818
- DOI: 10.1371/journal.pone.0023975
Differential temporal and spatial progerin expression during closure of the ductus arteriosus in neonates
Erratum in
- PLoS One. 2011;6(9). doi: 10.1371/annotation/c0198e87-d087-444c-8be9-780adb1582be
Abstract
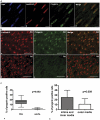
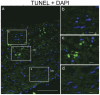
Closure of the ductus arteriosus (DA) at birth is essential for the transition from fetal to postnatal life. Before birth the DA bypasses the uninflated lungs by shunting blood from the pulmonary trunk into the systemic circulation. The molecular mechanism underlying DA closure and degeneration has not been fully elucidated, but is associated with apoptosis and cytolytic necrosis in the inner media and intima. We detected features of histology during DA degeneration that are comparable to Hutchinson Gilford Progeria syndrome and ageing. Immunohistochemistry on human fetal and neonatal DA, and aorta showed that lamin A/C was expressed in all layers of the vessel wall. As a novel finding we report that progerin, a splicing variant of lamin A/C was expressed almost selectively in the normal closing neonatal DA, from which we hypothesized that progerin is involved in DA closure. Progerin was detected in 16.2%±7.2 cells of the DA. Progerin-expressing cells were predominantly located in intima and inner media where cytolytic necrosis accompanied by apoptosis will develop. Concomitantly we found loss of α-smooth muscle actin as well as reduced lamin A/C expression compared to the fetal and non-closing DA. In cells of the adjacent aorta, that remains patent, progerin expression was only sporadically detected in 2.5%±1.5 of the cells. Data were substantiated by the detection of mRNA of progerin in the neonatal DA but not in the aorta, by PCR and sequencing analysis. The fetal DA and the non-closing persistent DA did not present with progerin expressing cells. Our analysis revealed that the spatiotemporal expression of lamin A/C and progerin in the neonatal DA was mutually exclusive. We suggest that activation of LMNA alternative splicing is involved in vascular remodeling in the circulatory system during normal neonatal DA closure.
Conflict of interest statement
Figures



References
-
- Bokenkamp R, DeRuiter MC, van Munsteren C, Gittenberger-de Groot AC. Insights into the Pathogenesis and Genetic Background of Patency of the Ductus Arteriosus. Neonatology. 2009;98:6–17. - PubMed
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. - PubMed
-
- Gittenberger-de Groot AC, van Ertbruggen I, Moulaert AJMG, Harinck E. The ductus arteriosus in the preterm infant: histological and clinical observation. J Pediatr. 1980;96:88–93. - PubMed
-
- Slomp J, van Munsteren JC, Poelmann RE, de Reeder EG, Bogers AJJC, et al. Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis. 1992;93:25–39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

