Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3
- PMID: 21916500
- PMCID: PMC3359705
- DOI: 10.1021/jo201478d
Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3
Abstract
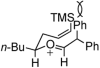
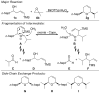
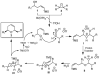
Catalytic quantities of bismuth(III) triflate efficiently initiate the rearrangement of epoxides to aldehydes, which subsequently react with (Z)-δ-hydroxyalkenylsilanes to afford 2,6-disubstituted 3,6-dihydro-2H-pyrans. Isolated yields of desired products using Bi(OTf)(3) were compared with yields obtained when the reactions were run with TfOH and TMSOTf in the presence and absence of several additives. These studies, as well as NMR spectroscopic analyses, indicate an initial Lewis acid/base interaction between Bi(OTf)(3) and substrates providing TfOH in situ.
Figures
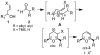
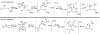
Similar articles
-
Diastereoselective synthesis of substituted dihydropyrans via an oxonium-ene cyclization reaction.Org Biomol Chem. 2012 Nov 21;10(43):8730-8. doi: 10.1039/c2ob26088c. Org Biomol Chem. 2012. PMID: 23037969
-
Solvent-dependent divergent functions of Sc(OTf)₃ in stereoselective epoxide-opening spiroketalizations.Org Lett. 2014 May 2;16(9):2474-7. doi: 10.1021/ol500853q. Epub 2014 Apr 17. Org Lett. 2014. PMID: 24742081 Free PMC article.
-
Lewis acid mediated intramolecular C-C bond formation of alkyne-epoxide leading to six-membered nitrogen and oxygen heterocycles.J Org Chem. 2014 May 2;79(9):4119-24. doi: 10.1021/jo402550e. Epub 2014 Apr 18. J Org Chem. 2014. PMID: 24720735
-
Chemistry of fullerene epoxides: synthesis, structure, and nucleophilic substitution-addition reactivity.Molecules. 2012 May 25;17(6):6395-414. doi: 10.3390/molecules17066395. Molecules. 2012. PMID: 22634847 Free PMC article. Review.
-
Quantum mechanical investigations of organocatalysis: mechanisms, reactivities, and selectivities.Chem Rev. 2011 Aug 10;111(8):5042-137. doi: 10.1021/cr100212h. Epub 2011 Jun 28. Chem Rev. 2011. PMID: 21707120 Free PMC article. Review. No abstract available.
Cited by
-
Atom economical, one-pot, three-reaction cascade to novel tricyclic 2,4-dihydro-1H-benzo[f]isochromenes.Org Lett. 2013 Aug 16;15(16):4070-3. doi: 10.1021/ol401600j. Epub 2013 Aug 1. Org Lett. 2013. PMID: 23901903 Free PMC article.
-
Mechanism of Friedel-Crafts Acylation Using Metal Triflate in Deep Eutectic Solvents: An Experimental and Computational Study.ACS Omega. 2022 Dec 16;8(1):271-278. doi: 10.1021/acsomega.2c03944. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643563 Free PMC article.
-
Synthesis of functionalised isochromans: epoxides as aldehyde surrogates in hexafluoroisopropanol.Chem Sci. 2023 Feb 15;14(11):2983-2989. doi: 10.1039/d2sc06692k. eCollection 2023 Mar 15. Chem Sci. 2023. PMID: 36937595 Free PMC article.
-
Vinylsilanes in Highly Diastereo- and Regio-Selective Synthesis of Dihydropyrans.ACS Omega. 2019 Feb 4;4(2):2630-2636. doi: 10.1021/acsomega.8b03635. eCollection 2019 Feb 28. ACS Omega. 2019. PMID: 31459498 Free PMC article.
-
Ring-Closing Synthesis of Dibenzothiophene Sulfonium Salts and Their Use as Leaving Groups for Aromatic 18F-Fluorination.J Am Chem Soc. 2018 Sep 5;140(35):11125-11132. doi: 10.1021/jacs.8b06730. Epub 2018 Aug 22. J Am Chem Soc. 2018. PMID: 30132661 Free PMC article.
References
-
- Bothwell JM, Krabbe SW, Mohan RS. Chem Soc Rev. 2011;40:4649–4704. - PubMed
- Sandhu S, Sandhu JS. Rasãyan J Chem. 2011;4:73–85.
- Salvador JAR, Pinto RMA, Silvestre SM. Curr Org Synth. 2009;6:426–470.
- Hua R. Curr Org Synth. 2008;5:1–27.
- Komatsu N. In: Organobismuth Chemistry. Suzuki H, Matano Y, editors. Elsevier; New York: 2001. pp. 371–440.
- Gaspard-Iloughmane H, Le Roux C. Eur J Org Chem. 2004:2517–2532.
- Leonard NW, Wieland LC, Mohan RS. Tetrahedron. 2002;58:8373–8397.
-
- Komatsu N, Uda M, Suzuki H, Takahashi T, Domae T, Wada M. Tetrahedron Lett. 1997;38:7215–7218.
-
- Wada M, Nagayama S, Mizutani K, Hiroi R, Miyoshi N. Chem Lett. 2002:248–249.
-
- Garrigues B, Gonzaga F, Robert H, Dubac J. J Org Chem. 1997;62:4880–4882.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

