A field trial to assess a blood-stage malaria vaccine
- PMID: 21916638
- PMCID: PMC3242358
- DOI: 10.1056/NEJMoa1008115
A field trial to assess a blood-stage malaria vaccine
Abstract
Background: Blood-stage malaria vaccines are intended to prevent clinical disease. The malaria vaccine FMP2.1/AS02(A), a recombinant protein based on apical membrane antigen 1 (AMA1) from the 3D7 strain of Plasmodium falciparum, has previously been shown to have immunogenicity and acceptable safety in Malian adults and children.
Methods: In a double-blind, randomized trial, we immunized 400 Malian children with either the malaria vaccine or a control (rabies) vaccine and followed them for 6 months. The primary end point was clinical malaria, defined as fever and at least 2500 parasites per cubic millimeter of blood. A secondary end point was clinical malaria caused by parasites with the AMA1 DNA sequence found in the vaccine strain.
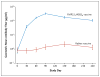
Results: The cumulative incidence of the primary end point was 48.4% in the malaria-vaccine group and 54.4% in the control group; efficacy against the primary end point was 17.4% (hazard ratio for the primary end point, 0.83; 95% confidence interval [CI], 0.63 to 1.09; P=0.18). Efficacy against the first and subsequent episodes of clinical malaria, as defined on the basis of various parasite-density thresholds, was approximately 20%. Efficacy against clinical malaria caused by parasites with AMA1 corresponding to that of the vaccine strain was 64.3% (hazard ratio, 0.36; 95% CI, 0.08 to 0.86; P=0.03). Local reactions and fever after vaccination were more frequent with the malaria vaccine.
Conclusions: On the basis of the primary end point, the malaria vaccine did not provide significant protection against clinical malaria, but on the basis of secondary results, it may have strain-specific efficacy. If this finding is confirmed, AMA1 might be useful in a multicomponent malaria vaccine. (Funded by the National Institute of Allergy and Infectious Diseases and others; ClinicalTrials.gov number, NCT00460525.).
Figures

References
-
- Plowe CV, Alonso P, Hoffman SL. The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malaria. J Infect Dis. 2009;200:1646–9. - PubMed
-
- Heppner DG, Jr, Kester KE, Ockenhouse CF, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23:2243–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
