Adenosine receptor signaling modulates permeability of the blood-brain barrier
- PMID: 21917810
- PMCID: PMC3328085
- DOI: 10.1523/JNEUROSCI.3337-11.2011
Adenosine receptor signaling modulates permeability of the blood-brain barrier
Abstract
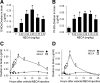
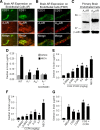
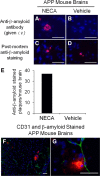
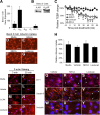
The blood-brain barrier (BBB) is comprised of specialized endothelial cells that form the capillary microvasculature of the CNS and is essential for brain function. It also poses the greatest impediment in the treatment of many CNS diseases because it commonly blocks entry of therapeutic compounds. Here we report that adenosine receptor (AR) signaling modulates BBB permeability in vivo. A(1) and A(2A) AR activation facilitated the entry of intravenously administered macromolecules, including large dextrans and antibodies to β-amyloid, into murine brains. Additionally, treatment with an FDA-approved selective A(2A) agonist, Lexiscan, also increased BBB permeability in murine models. These changes in BBB permeability are dose-dependent and temporally discrete. Transgenic mice lacking A(1) or A(2A) ARs showed diminished dextran entry into the brain after AR agonism. Following treatment with a broad-spectrum AR agonist, intravenously administered anti-β-amyloid antibody was observed to enter the CNS and bind β-amyloid plaques in a transgenic mouse model of Alzheimer's disease (AD). Selective AR activation resulted in cellular changes in vitro including decreased transendothelial electrical resistance, increased actinomyosin stress fiber formation, and alterations in tight junction molecules. These results suggest that AR signaling can be used to modulate BBB permeability in vivo to facilitate the entry of potentially therapeutic compounds into the CNS. AR signaling at brain endothelial cells represents a novel endogenous mechanism of modulating BBB permeability. We anticipate these results will aid in drug design, drug delivery and treatment options for neurological diseases such as AD, Parkinson's disease, multiple sclerosis and cancers of the CNS.
Figures

Comment in
-
New methods to permeabilize the blood-brain barrier.Nat Rev Neurol. 2011 Oct 18;7(11):597. doi: 10.1038/nrneurol.2011.161. Nat Rev Neurol. 2011. PMID: 22009285 No abstract available.
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Bartus RT, Elliott P, Hayward N, Dean R, McEwen EL, Fisher SK. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology. 1996;33:270–278. - PubMed
-
- Borlongan CV, Emerich DF. Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Res Bull. 2003;60:297–306. - PubMed
-
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
