The Wnt/beta-catenin asymmetry pathway patterns the atonal ortholog lin-32 to diversify cell fate in a Caenorhabditis elegans sensory lineage
- PMID: 21917811
- PMCID: PMC3183998
- DOI: 10.1523/JNEUROSCI.6504-10.2011
The Wnt/beta-catenin asymmetry pathway patterns the atonal ortholog lin-32 to diversify cell fate in a Caenorhabditis elegans sensory lineage
Abstract
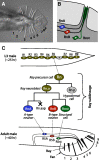
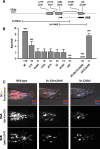
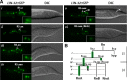
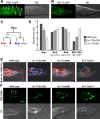
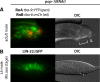
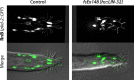
Each sensory ray of the Caenorhabditis elegans male tail comprises three distinct neuroglial cell types. These three cells descend from a single progenitor, the ray precursor cell, through several rounds of asymmetric division called the ray sublineage. Ray development requires the conserved atonal-family bHLH gene lin-32, which specifies the ray neuroblast and promotes the differentiation of its progeny. However, the mechanisms that allocate specific cell fates among these progeny are unknown. Here we show that the distribution of LIN-32 during the ray sublineage is markedly asymmetric, localizing to anterior daughter cells in two successive cell divisions. The anterior-posterior patterning of LIN-32 expression and of differentiated ray neuroglial fates is brought about by the Wnt/β-catenin asymmetry pathway, including the Wnt ligand LIN-44, its receptor LIN-17, and downstream components LIT-1 (NLK), SYS-1 (β-catenin), and POP-1 (TCF). LIN-32 asymmetry itself has an important role in patterning ray cell fates, because the failure to silence lin-32 expression in posterior cells disrupts development of this branch of the ray sublineage. Together, our results illustrate a mechanism whereby the regulated function of a proneural-class gene in a single neural lineage can both specify a neural precursor and actively pattern the fates of its progeny. Moreover, they reveal a central role for the Wnt/β-catenin asymmetry pathway in patterning neural and glial fates in a simple sensory lineage.
Figures


Similar articles
-
Re-programming of C. elegans male epidermal precursor fates by Wnt, Hox, and LIN-12/Notch activities.Dev Biol. 2010 Sep 1;345(1):1-11. doi: 10.1016/j.ydbio.2010.05.008. Epub 2010 May 15. Dev Biol. 2010. PMID: 20478294 Free PMC article.
-
Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1.Development. 2007 Jul;134(14):2685-95. doi: 10.1242/dev.008268. Epub 2007 Jun 13. Development. 2007. PMID: 17567664
-
The N- or C-terminal domains of DSH-2 can activate the C. elegans Wnt/beta-catenin asymmetry pathway.Dev Biol. 2009 Apr 15;328(2):234-44. doi: 10.1016/j.ydbio.2009.01.017. Epub 2009 Jan 23. Dev Biol. 2009. PMID: 19298786 Free PMC article.
-
The long and the short of Wnt signaling in C. elegans.Curr Opin Genet Dev. 2008 Aug;18(4):362-7. doi: 10.1016/j.gde.2008.06.006. Epub 2008 Jul 28. Curr Opin Genet Dev. 2008. PMID: 18625312 Free PMC article. Review.
-
A new look at TCF and beta-catenin through the lens of a divergent C. elegans Wnt pathway.Dev Cell. 2009 Jul;17(1):27-34. doi: 10.1016/j.devcel.2009.07.002. Dev Cell. 2009. PMID: 19619489 Free PMC article. Review.
Cited by
-
Caenorhabditis elegans male sensory-motor neurons and dopaminergic support cells couple ejaculation and post-ejaculatory behaviors.Elife. 2014 Jun 10;3:e02938. doi: 10.7554/eLife.02938. Elife. 2014. PMID: 24915976 Free PMC article.
-
Tcf7l2 is required for left-right asymmetric differentiation of habenular neurons.Curr Biol. 2014 Oct 6;24(19):2217-27. doi: 10.1016/j.cub.2014.08.006. Epub 2014 Sep 4. Curr Biol. 2014. PMID: 25201686 Free PMC article.
-
HES-Mediated Repression of Pten in Caenorhabditis elegans.G3 (Bethesda). 2015 Oct 4;5(12):2619-28. doi: 10.1534/g3.115.019463. G3 (Bethesda). 2015. PMID: 26438299 Free PMC article.
-
Atypical Transcriptional Activation by TCF via a Zic Transcription Factor in C. elegans Neuronal Precursors.Dev Cell. 2015 Jun 22;33(6):737-45. doi: 10.1016/j.devcel.2015.04.018. Epub 2015 Jun 11. Dev Cell. 2015. PMID: 26073017 Free PMC article.
-
A bHLH Code for Sexually Dimorphic Form and Function of the C. elegans Somatic Gonad.Curr Biol. 2017 Jun 19;27(12):1853-1860.e5. doi: 10.1016/j.cub.2017.05.059. Epub 2017 Jun 8. Curr Biol. 2017. PMID: 28602651 Free PMC article.
References
-
- Barr MM, Garcia LR. Male behavior. Wormbook. 2006. E-book available at www.wormbook.org. - PMC - PubMed
-
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. - PubMed
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
