Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (phase Iiib ALLOW study)
- PMID: 21917824
- PMCID: PMC3233696
- DOI: 10.1136/annrheumdis-2011-200344
Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (phase Iiib ALLOW study)
Abstract
Objectives: To assess the effect of a temporary interruption in subcutaneous (SC) abatacept on immunogenicity, safety and efficacy in patients with active rheumatoid arthritis despite methotrexate in a phase III trial.
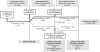
Methods: Following a 12-week open-label introduction (period I; intravenous abatacept loading dose and weekly fixed-dose SC abatacept 125 mg), patients were randomised 2:1 to double-blind SC placebo or SC abatacept for 12 weeks (period II). At the end of period II, patients receiving SC abatacept continued treatment and patients on placebo were reintroduced to SC abatacept (12-week open-label period III). The co-primary end points were ELISA-detected immunogenicity rate and safety at the end of period II. Efficacy was also monitored.
Results: Of 167 patients entering period I, 72% qualified for period II; during periods II and III, three patients discontinued treatment. Mean (SD) disease duration was 6.6 (6.5) years and Disease Activity Score 28 was 4.8 (0.8). The primary end point was met, with a non-significant increase in immunogenicity upon withdrawal (7/73 placebo vs 0/38 abatacept in period II; p=0.119) which was reversed upon reintroduction of SC abatacept (2/73 vs 1/38, end period III). Safety was comparable regardless of withdrawal, with no unexpected events upon reintroduction. Two patients experienced reactions at the SC injection site. On withdrawal, patients experienced slight worsening in efficacy which improved following reintroduction.
Conclusions: Overall immunogenicity to SC abatacept is low, consistent with intravenous abatacept, and is not significantly affected by a 3-month interruption and reintroduction. This stop-start schedule was well tolerated, with little impact on safety and efficacy. These are important considerations for the clinical use of SC abatacept.
Clinicaltrials: gov Identifier NCT00533897.
Conflict of interest statement
Figures


References
-
- Haggerty HG, Abbott MA, Reilly TP, et al. Evaluation of immunogenicity of the T cell costimulation modulator abatacept in patients treated for rheumatoid arthritis. J Rheumatol 2007;34:2365–73 - PubMed
-
- Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant 2005;20Suppl 6:vi3–9 - PubMed
-
- Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, et al. Immunogenicity of anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol 2010;38:82–9 - PubMed
-
- Janssen Biotech, Inc Remicade prescribing information. http://www.remicade.com/remicade/assets/HCP_PPI.pdf (accessed February 2011)
-
- Vultaggio A, Matucci A, Nencini F, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010;65:657–61 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

