Cardiac PET/CT for the evaluation of known or suspected coronary artery disease
- PMID: 21918042
- PMCID: PMC3173713
- DOI: 10.1148/rg.315115056
Cardiac PET/CT for the evaluation of known or suspected coronary artery disease
Abstract
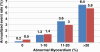
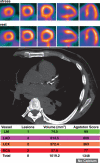
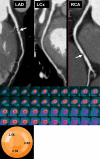
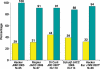
Positron emission tomography (PET) is increasingly being applied in the evaluation of myocardial perfusion. Cardiac PET can be performed with an increasing variety of cyclotron- and generator-produced radiotracers. Compared with single photon emission computed tomography, PET offers lower radiation exposure, fewer artifacts, improved spatial resolution, and, most important, improved diagnostic performance. With its capacity to quantify rest-peak stress left ventricular systolic function as well as coronary flow reserve, PET is superior to other methods for the detection of multivessel coronary artery disease and, potentially, for risk stratification. Coronary artery calcium scoring may be included for further risk stratification in patients with normal perfusion imaging findings. Furthermore, PET allows quantification of absolute myocardial perfusion, which also carries substantial prognostic value. Hybrid PET-computed tomography scanners allow functional evaluation of myocardial perfusion combined with anatomic characterization of the epicardial coronary arteries, thereby offering great potential for both diagnosis and management. Additional studies to further validate the prognostic value and cost effectiveness of PET are warranted.
Figures

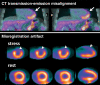
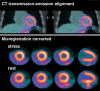


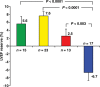
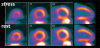




References
-
- Schelbert HR. Evaluation of myocardial blood flow in cardiac disease. In: Skorton DJ, Schelbert HR, Wolf GL, Brundage BH, eds. Cardiac imaging: a companion to Braunwald's heart disease. Philadelphia, Pa: Saunders, 1991; 1093–1112
-
- Shelton ME, Green MA, Mathias CJ, Welch MJ, Bergmann SR. Kinetics of copper-PTSM in isolated hearts: a novel tracer for measuring blood flow with positron emission tomography. J Nucl Med 1989;30(11):1843–1847 - PubMed
-
- Shelton ME, Green MA, Mathias CJ, Welch MJ, Bergmann SR. Assessment of regional myocardial and renal blood flow with copper-PTSM and positron emission tomography. Circulation 1990;82(3): 990–997 - PubMed
-
- Beanlands RS, Muzik O, Mintun M, et al. The kinetics of copper-62-PTSM in the normal human heart. J Nucl Med 1992;33(5):684–690 - PubMed
-
- Herrero P, Hartman JJ, Green MA, et al. Regional myocardial perfusion assessed with generator-produced copper-62-PTSM and PET. J Nucl Med 1996;37(8):1294–1300 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

