Galphas-biased beta2-adrenergic receptor signaling from restoring synchronous contraction in the failing heart
- PMID: 21918105
- PMCID: PMC3329869
- DOI: 10.1126/scitranslmed.3001909
Galphas-biased beta2-adrenergic receptor signaling from restoring synchronous contraction in the failing heart
Abstract
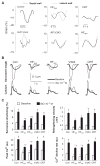
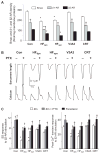
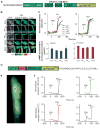
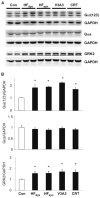
Cardiac resynchronization therapy (CRT), in which both ventricles are paced to recoordinate contraction in hearts that are dyssynchronous from conduction delay, is the only heart failure (HF) therapy to date to clinically improve acute and chronic function while also lowering mortality. CRT acutely enhances chamber mechanical efficiency but chronically alters myocyte signaling, including improving β-adrenergic receptor reserve. We speculated that the latter would identify unique CRT effects that might themselves be effective for HF more generally. HF was induced in dogs by 6 weeks of atrial rapid pacing with (HFdys, left bundle ablated) or without (HFsyn) dyssynchrony. We used dyssynchronous followed by resynchronized tachypacing (each 3 weeks) for CRT. Both HFdys and HFsyn myocytes had similarly depressed rest and β-adrenergic receptor sarcomere and calcium responses, particularly the β2-adrenergic response, whereas cells subjected to CRT behaved similarly to those from healthy controls. CRT myocytes exhibited suppressed Gαi signaling linked to increased regulator of G protein (heterotrimeric guanine nucleotide-binding protein) signaling (RGS2, RGS3), yielding Gαs-biased β2-adrenergic responses. This included increased adenosine cyclic AMP responsiveness and activation of sarcoplasmic reticulum-localized protein kinase A. Human CRT responders also showed up-regulated myocardial RGS2 and RGS3. Inhibition of Gαi (with pertussis toxin, RGS3, or RGS2 transfection), stimulation with a Gαs-biased β2 agonist (fenoterol), or transient (2-week) exposure to dyssynchrony restored β-adrenergic receptor responses in HFsyn to the values obtained after CRT. These results identify a key pathway that is triggered by restoring contractile synchrony and that may represent a new therapeutic approach for a broad population of HF patients.
Conflict of interest statement
Figures

Similar articles
-
Mechanisms of enhanced beta-adrenergic reserve from cardiac resynchronization therapy.Circulation. 2009 Mar 10;119(9):1231-40. doi: 10.1161/CIRCULATIONAHA.108.774752. Epub 2009 Feb 23. Circulation. 2009. PMID: 19237665 Free PMC article.
-
Cardiac resynchronization therapy restores sympathovagal balance in the failing heart by differential remodeling of cholinergic signaling.Circ Res. 2015 May 8;116(10):1691-9. doi: 10.1161/CIRCRESAHA.116.305268. Epub 2015 Mar 2. Circ Res. 2015. PMID: 25733594 Free PMC article.
-
Enhanced G(i) signaling selectively negates beta2-adrenergic receptor (AR)--but not beta1-AR-mediated positive inotropic effect in myocytes from failing rat hearts.Circulation. 2003 Sep 30;108(13):1633-9. doi: 10.1161/01.CIR.0000087595.17277.73. Epub 2003 Sep 15. Circulation. 2003. PMID: 12975249
-
Electromechanical dyssynchrony and resynchronization of the failing heart.Circ Res. 2013 Aug 30;113(6):765-76. doi: 10.1161/CIRCRESAHA.113.300270. Circ Res. 2013. PMID: 23989718 Free PMC article. Review.
-
Electrical remodeling in dyssynchrony and resynchronization.J Cardiovasc Transl Res. 2012 Apr;5(2):170-9. doi: 10.1007/s12265-012-9348-9. Epub 2012 Jan 21. J Cardiovasc Transl Res. 2012. PMID: 22271011 Review.
Cited by
-
Pathological hypertrophy reverses β2-adrenergic receptor-induced angiogenesis in mouse heart.Physiol Rep. 2015 Mar;3(3):e12340. doi: 10.14814/phy2.12340. Physiol Rep. 2015. PMID: 25780088 Free PMC article.
-
Metabolic remodeling in moderate synchronous versus dyssynchronous pacing-induced heart failure: integrated metabolomics and proteomics study.PLoS One. 2015 Mar 19;10(3):e0118974. doi: 10.1371/journal.pone.0118974. eCollection 2015. PLoS One. 2015. PMID: 25790351 Free PMC article.
-
Phospho-Proteomic Analysis of Cardiac Dyssynchrony and Resynchronization Therapy.Proteomics. 2018 Oct;18(19):e1800079. doi: 10.1002/pmic.201800079. Epub 2018 Aug 30. Proteomics. 2018. PMID: 30129105 Free PMC article.
-
Chronic Atrial and Ventricular Pacing in the Mouse.Circ Heart Fail. 2019 Feb;12(2):e005655. doi: 10.1161/CIRCHEARTFAILURE.118.005655. Circ Heart Fail. 2019. PMID: 30764638 Free PMC article.
-
Reverse remodeling in heart failure--mechanisms and therapeutic opportunities.Nat Rev Cardiol. 2011 Dec 6;9(3):147-57. doi: 10.1038/nrcardio.2011.172. Nat Rev Cardiol. 2011. PMID: 22143079 Review.
References
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation. 2010;121:948–954. - PubMed
-
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators, The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. - PubMed
-
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J. 2006;27:1928–1932. - PubMed
-
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators, Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. - PubMed
-
- Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation, Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK073368/DK/NIDDK NIH HHS/United States
- R01 HL089297/HL/NHLBI NIH HHS/United States
- P01 HL077180/HL/NHLBI NIH HHS/United States
- R01-DK073368/DK/NIDDK NIH HHS/United States
- R01-HL-089297/HL/NHLBI NIH HHS/United States
- T32-HL0072/HL/NHLBI NIH HHS/United States
- R01-GM085058/GM/NIGMS NIH HHS/United States
- F31 GM087079/GM/NIGMS NIH HHS/United States
- DP1OD006419/OD/NIH HHS/United States
- DP1 OD006419/OD/NIH HHS/United States
- R01 GM085058/GM/NIGMS NIH HHS/United States
- F31-GM087079/GM/NIGMS NIH HHS/United States
- P01-HL077180/HL/NHLBI NIH HHS/United States
- R01 HL082846/HL/NHLBI NIH HHS/United States
- R01-HL-053432/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

