Deletion of Syk in neutrophils prevents immune complex arthritis
- PMID: 21918195
- PMCID: PMC3186826
- DOI: 10.4049/jimmunol.1100341
Deletion of Syk in neutrophils prevents immune complex arthritis
Abstract
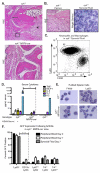
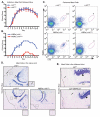
The K/BxN serum transfer model of arthritis is critically dependent on FcγR signaling events mediated by spleen tyrosine kinase (Syk). However, the specific cell types in which this signaling is required are not known. We report that deletion of Syk in neutrophils, achieved using syk(f/f) MRP8-cre(+) mice, blocks disease development in serum transfer arthritis. The syk(f/f) MRP8-cre(+) mice display absent joint disease and reduced deposition of pathogenic anti-glucose-6-phosphate isomerase Abs in the joint (with a reciprocal accumulation of these Abs in the peripheral circulation). Additionally, syk(f/f) MRP8-cre(+) mice manifest poor edema formation within 3 h after formation of cutaneous immune complexes (Arthus reaction). Together, this suggests that neutrophil-dependent recognition of immune complexes contributes significantly to changes in vascular permeability during the early phases of immune complex disease. Using mixed chimeric mice, containing both wild-type and syk(f/f) MRP8-cre(+) neutrophils, we find no impairment in recruitment of Syk-deficient neutrophils to the inflamed joint, but they fail to become primed, demonstrating lower cytokine production after removal from the joint. They also display an increased apoptotic rate compared with wild-type cells in the same joint. Mast cell-deficient c-kit(sh/sh) mice developed robust arthritis after serum transfer whereas c-kit(W/Wv) mice did not, suggesting that previous conclusions concerning the central role of mast cells in this model may need to be revised. Basophil-deficient mice also responded normally to K/BxN serum transfer. These results demonstrate that Syk-dependent signaling in neutrophils alone is critically required for arthritis development in the serum transfer model.
Figures






References
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. - PubMed
-
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10:427–439. - PubMed
-
- Cassatella MA, Locati M, Mantovani A. Never underestimate the power of a neutrophil. Immunity. 2009;31:698–700. - PubMed
-
- Cascao R, Rosario HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun. Rev. 2010;9:531–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

