Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects
- PMID: 21918504
- PMCID: PMC3306868
- DOI: 10.1038/npp.2011.200
Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects
Abstract
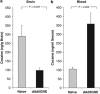
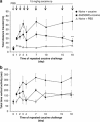
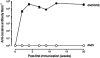
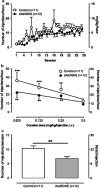
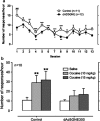
Immunotherapy is a promising treatment for drug addiction. However, insufficient immune responses to vaccines in most subjects pose a challenge. In this study, we tested the efficacy of a new cocaine vaccine (dAd5GNE) in antagonizing cocaine addiction-related behaviors in rats. This vaccine used a disrupted serotype 5 adenovirus (Ad) gene transfer vector coupled to a third-generation cocaine hapten, termed GNE (6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carboxamido-hexanoic acid). Three groups of rats were immunized with dAd5GNE. One group was injected with (3)H-cocaine, and radioactivity in the blood and brain was determined. A second group was tested for cocaine-induced locomotor sensitization. A third group was examined for cocaine self-administration, extinction, and reinstatement of responding for cocaine. Antibody titers were determined at various time-points. In each experiment, we added a control group that was immunized with dAd5 without a hapten. The vaccination with dAd5GNE produced long-lasting high titers (>10(5)) of anti-cocaine antibodies in all of the rats. The vaccination inhibited cocaine-induced hyperlocomotor activity and sensitization. Vaccinated rats acquired cocaine self-administration, but they showed less motivation to self-administer cocaine under a progressive-ratio schedule than control rats. When cocaine was not available in a session, control rats exhibited 'extinction burst' responding, whereas vaccinated rats did not. Moreover, when primed with cocaine, vaccinated rats did not reinstate responding, suggesting a blockade of cocaine-seeking behavior. These data strongly suggest that our dAd5GNE vector-based vaccine may be effective in treating cocaine abuse and addiction.
Figures

Comment in
-
Thinking outside the synapse: pharmacokinetic-based medications for cocaine addiction.Neuropsychopharmacology. 2012 Apr;37(5):1079-80. doi: 10.1038/npp.2011.269. Epub 2011 Nov 9. Neuropsychopharmacology. 2012. PMID: 22071873 Free PMC article. No abstract available.
Similar articles
-
Locomotor activity and cocaine-seeking behavior during acquisition and reinstatement of operant self-administration behavior in rats.Behav Brain Res. 2005 May 28;160(2):250-9. doi: 10.1016/j.bbr.2004.12.005. Epub 2005 Jan 12. Behav Brain Res. 2005. PMID: 15863221
-
Active vaccination reduces reinforcing effects of MDPV in male Sprague-Dawley rats trained to self-administer cocaine.Psychopharmacology (Berl). 2020 Sep;237(9):2613-2620. doi: 10.1007/s00213-020-05558-0. Epub 2020 Jun 4. Psychopharmacology (Berl). 2020. PMID: 32500210 Free PMC article.
-
Effects of a histone deacetylase 3 inhibitor on extinction and reinstatement of cocaine self-administration in rats.Psychopharmacology (Berl). 2019 Jan;236(1):517-529. doi: 10.1007/s00213-018-5122-2. Epub 2018 Nov 28. Psychopharmacology (Berl). 2019. PMID: 30488346 Free PMC article.
-
Anti-cocaine vaccine based on coupling a cocaine analog to a disrupted adenovirus.CNS Neurol Disord Drug Targets. 2011 Dec;10(8):899-904. doi: 10.2174/187152711799219334. CNS Neurol Disord Drug Targets. 2011. PMID: 22229312 Free PMC article. Review.
-
The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures.Psychopharmacology (Berl). 2013 Mar;226(1):113-25. doi: 10.1007/s00213-012-2899-2. Epub 2012 Oct 21. Psychopharmacology (Berl). 2013. PMID: 23086021 Free PMC article. Review.
Cited by
-
Environmental, genetic and epigenetic contributions to cocaine addiction.Neuropsychopharmacology. 2018 Jun;43(7):1471-1480. doi: 10.1038/s41386-018-0008-x. Epub 2018 Feb 5. Neuropsychopharmacology. 2018. PMID: 29453446 Free PMC article. Review.
-
Four decades of adenovirus gene transfer vectors: History and current use.Mol Ther. 2025 May 7;33(5):2192-2204. doi: 10.1016/j.ymthe.2025.03.062. Epub 2025 Apr 2. Mol Ther. 2025. PMID: 40181546
-
Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter.Neuropsychopharmacology. 2013 Oct;38(11):2170-8. doi: 10.1038/npp.2013.114. Epub 2013 May 10. Neuropsychopharmacology. 2013. PMID: 23660705 Free PMC article.
-
Combined cocaine hydrolase gene transfer and anti-cocaine vaccine synergistically block cocaine-induced locomotion.PLoS One. 2012;7(8):e43536. doi: 10.1371/journal.pone.0043536. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912888 Free PMC article.
-
Improving translation of animal models of addiction and relapse by reverse translation.Nat Rev Neurosci. 2020 Nov;21(11):625-643. doi: 10.1038/s41583-020-0378-z. Epub 2020 Oct 6. Nat Rev Neurosci. 2020. PMID: 33024318 Review.
References
-
- Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24:3232–3240. - PubMed
-
- Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, et al. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin. 2009;5:214–229. - PubMed
-
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature. 1974;252:708–710. - PubMed
-
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. - PubMed
-
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

