Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior
- PMID: 21918508
- PMCID: PMC3238082
- DOI: 10.1038/npp.2011.205
Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior
Abstract
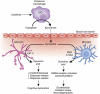
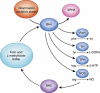
The potential contribution of chronic inflammation to the development of neuropsychiatric disorders such as major depression has received increasing attention. Elevated biomarkers of inflammation, including inflammatory cytokines and acute-phase proteins, have been found in depressed patients, and administration of inflammatory stimuli has been associated with the development of depressive symptoms. Data also have demonstrated that inflammatory cytokines can interact with multiple pathways known to be involved in the development of depression, including monoamine metabolism, neuroendocrine function, synaptic plasticity, and neurocircuits relevant to mood regulation. Further understanding of mechanisms by which cytokines alter behavior have revealed a host of pharmacologic targets that may be unique to the impact of inflammation on behavior and may be especially relevant to the treatment and prevention of depression in patients with evidence of increased inflammation. Such targets include the inflammatory signaling pathways cyclooxygenase, p38 mitogen-activated protein kinase, and nuclear factor-κB, as well as the metabolic enzyme, indoleamine-2,3-dioxygenase, which breaks down tryptophan into kynurenine. Other targets include the cytokines themselves in addition to chemokines, which attract inflammatory cells from the periphery to the brain. Psychosocial stress, diet, obesity, a leaky gut, and an imbalance between regulatory and pro-inflammatory T cells also contribute to inflammation and may serve as a focus for preventative strategies relevant to both the development of depression and its recurrence. Taken together, identification of mechanisms by which cytokines influence behavior may reveal a panoply of personalized treatment options that target the unique contributions of the immune system to depression.
Figures


References
-
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. - PubMed
-
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. - PubMed
-
- Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33–38. - PubMed
-
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. - PubMed
Publication types
MeSH terms
Grants and funding
- M01 RR0039/RR/NCRR NIH HHS/United States
- R01 AT004698/AT/NCCIH NIH HHS/United States
- R01 MH083746/MH/NIMH NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- R01MH75102/MH/NIMH NIH HHS/United States
- R01MH075102/MH/NIMH NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- K23 MH091254/MH/NIMH NIH HHS/United States
- R01MH083746/MH/NIMH NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- R01AT004698/AT/NCCIH NIH HHS/United States
- R01 MH075102/MH/NIMH NIH HHS/United States
- R01MH087604/MH/NIMH NIH HHS/United States
- K23MH091254/MH/NIMH NIH HHS/United States
- R01 MH087604/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

